Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism
- PMID: 32867207
- PMCID: PMC7551070
- DOI: 10.3390/toxins12090550
Fraternine, a Novel Wasp Peptide, Protects against Motor Impairments in 6-OHDA Model of Parkinsonism
Abstract
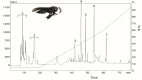
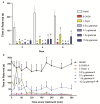
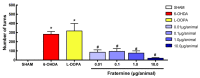
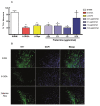
Parkinson's disease (PD) is a progressive neurodegenerative condition that affects the Central Nervous System (CNS). Insect venoms show high molecular variability and selectivity in the CNS of mammals and present potential for the development of new drugs for the treatment of PD. In this study, we isolated and identified a component of the venom of the social wasp Parachartergus fraternus and evaluated its neuroprotective activity in the murine model of PD. For this purpose, the venom was filtered and separated through HPLC; fractions were analyzed through mass spectrometry and the active fraction was identified as a novel peptide, called Fraternine. We performed two behavioral tests to evaluate motor discoordination, as well as an apomorphine-induced rotation test. We also conducted an immunohistochemical assay to assess protection in TH+ neurons in the Substantia Nigra (SN) region. Group treated with 10 μg/animal of Fraternine remained longer in the rotarod compared to the lesioned group. In the apomorphine test, Fraternine decreased the number of rotations between treatments. This dose also inhibited dopaminergic neuronal loss, as indicated by immunohistochemical analysis. This study identified a novel peptide able to prevent the death of dopaminergic neurons of the SN and recover motor deficit in a 6-OHDA-induced murine model of PD.
Keywords: Parkinson’s disease; antiparkinsonian activity; motor behavior; neuroprotective effect; wasp venom.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures

Similar articles
-
The Kv7/KCNQ channel blocker XE991 protects nigral dopaminergic neurons in the 6-hydroxydopamine rat model of Parkinson's disease.Brain Res Bull. 2018 Mar;137:132-139. doi: 10.1016/j.brainresbull.2017.11.011. Epub 2017 Nov 22. Brain Res Bull. 2018. PMID: 29174294
-
A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson's disease.Neuropharmacology. 2017 May 1;117:238-248. doi: 10.1016/j.neuropharm.2017.02.013. Epub 2017 Feb 20. Neuropharmacology. 2017. PMID: 28223210
-
Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents.Neuropharmacology. 2015 Dec;99:448-58. doi: 10.1016/j.neuropharm.2015.08.016. Epub 2015 Aug 14. Neuropharmacology. 2015. PMID: 26282118
-
Neuroprotective effects of garlic extract on dopaminergic neurons of substantia nigra in a rat model of Parkinson's disease: motor and non-motor outcomes.Metab Brain Dis. 2021 Jun;36(5):927-937. doi: 10.1007/s11011-021-00705-8. Epub 2021 Mar 3. Metab Brain Dis. 2021. PMID: 33656625
-
Neurotoxin-Induced Rodent Models of Parkinson's Disease: Benefits and Drawbacks.Neurotox Res. 2021 Jun;39(3):897-923. doi: 10.1007/s12640-021-00356-8. Epub 2021 Mar 25. Neurotox Res. 2021. PMID: 33765237 Review.
Cited by
-
Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective.Front Pharmacol. 2022 Sep 27;13:1001553. doi: 10.3389/fphar.2022.1001553. eCollection 2022. Front Pharmacol. 2022. PMID: 36238572 Free PMC article. Review.
-
Exploring the therapeutic potential of an antinociceptive and anti-inflammatory peptide from wasp venom.Sci Rep. 2023 Aug 1;13(1):12491. doi: 10.1038/s41598-023-38828-w. Sci Rep. 2023. PMID: 37528129 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

