Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia
- PMID: 32867218
- PMCID: PMC7564858
- DOI: 10.3390/genes11091008
Deciphering the Forebrain Disorder in a Chicken Model of Cerebral Hernia
Abstract
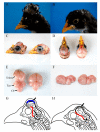
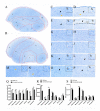
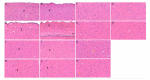
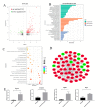
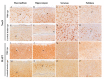
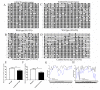
Cerebral hernia in crested chicken has been characterized as the protrusion of cerebral hemispheres into the unsealed skull for hundreds of years, since Charles Darwin. The development of deformed forebrain (telencephalon) of cerebral hernia remains largely unknown. Here, the unsealed frontal skull combined with misplaced sphenoid bone was observed and potentially associated with brain protuberance. The shifted pallidum, elongated hippocampus, expanded mesopallium and nidopallium, and reduced hyperpallium were observed in seven regions of the malformed telencephalon. The neurons were detected with nuclear pyknosis and decreased density. Astrocytes showed uneven distribution and disordered protuberances in hyperpallium and hippocampus. Transcriptome analyses of chicken telencephalon (cerebral hernia vs. control) revealed 547 differentially expressed genes (DEGs), mainly related to nervous system development, and immune system processes, including astrocyte marker gene GFAP, and neuron and astrocyte developmental gene S100A6. The upregulation of GFAP and S100A6 genes in abnormal telencephalon was correlated with reduced DNA methylation levels in the promoter regions. The morphological, cellular, and molecular variations in the shape, regional specification, and cellular states of malformed telencephalon potentially participate in brain plasticity and previously reported behavior changes. Chickens with cerebral hernia might be an interesting and valuable disease model to further explore the recognition, diagnosis, and therapy of cerebral hernia development of crested chickens and other species.
Keywords: DNA methylation; astrocyte; brain; cerebral hernia; chicken; neuron; sphenoid bone; telencephalon.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
HOXC10 intronic duplication is associated with unsealed skull and crest in crested chicken with cerebral hernia.Gene. 2022 Oct 5;840:146758. doi: 10.1016/j.gene.2022.146758. Epub 2022 Jul 26. Gene. 2022. PMID: 35905851
-
Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury.J Neurosci. 2020 Dec 9;40(50):9751-9771. doi: 10.1523/JNEUROSCI.0888-20.2020. Epub 2020 Nov 6. J Neurosci. 2020. PMID: 33158962 Free PMC article.
-
Inheritance and developmental pattern of cerebral hernia in the crested Polish chicken.J Exp Zool B Mol Dev Evol. 2012 Dec;318(8):613-20. doi: 10.1002/jez.b.22464. Epub 2012 Aug 21. J Exp Zool B Mol Dev Evol. 2012. PMID: 22907661
-
Distributions of two chicken bombesin receptors, bombesin receptor subtype-3.5 (chBRS-3.5) and gastrin-releasing peptide receptor (chGRP-R) mRNAS in the chicken telencephalon.Neuroscience. 2004;125(3):569-82. doi: 10.1016/j.neuroscience.2004.01.057. Neuroscience. 2004. PMID: 15099671
-
Forebrain glutamatergic neuron-specific Ctcf deletion induces reactive microgliosis and astrogliosis with neuronal loss in adult mouse hippocampus.BMB Rep. 2021 Jun;54(6):317-322. doi: 10.5483/BMBRep.2021.54.6.265. BMB Rep. 2021. PMID: 33612151 Free PMC article.
Cited by
-
Dumbbell-shaped brains of Polish crested chickens as a model system for the evolution of novel brain morphologies.J Anat. 2023 Sep;243(3):421-430. doi: 10.1111/joa.13883. Epub 2023 May 10. J Anat. 2023. PMID: 37165612 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

