The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose
- PMID: 32867240
- PMCID: PMC7504415
- DOI: 10.3390/molecules25173913
The Glycan Structure of T. cruzi mucins Depends on the Host. Insights on the Chameleonic Galactose
Abstract
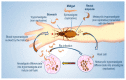
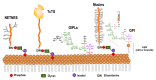
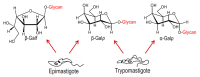
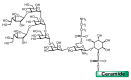
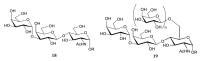
Trypanosoma cruzi, the protozoa that causes Chagas disease in humans, is transmitted by insects from the Reduviidae family. The parasite has developed the ability to change the structure of the surface molecules, depending on the host. Among them, the mucins are the most abundant glycoproteins. Structural studies have focused on the epimastigotes and metacyclic trypomastigotes that colonize the insect, and on the mammal trypomastigotes. The carbohydrate in the mucins fulfills crucial functions, the most important of which being the accepting of sialic acid from the host, a process catalyzed by the unique parasite trans-sialidase. The sialylation of the parasite influences the immune response on infection. The O-linked sugars have characteristics that differentiate them from human mucins. One of them is the linkage to the polypeptide chain by the hexosamine, GlcNAc, instead of GalNAc. The main monosaccharide in the mucins oligosaccharides is galactose, and this may be present in three configurations. Whereas β-d-galactopyranose (β-Galp) was found in the insect and the human stages of Trypanosoma cruzi, β-d-galactofuranose (β-Galf) is present only in the mucins of some strains of epimastigotes and α-d-galactopyranose (α-Galp) characterizes the mucins of the bloodstream trypomastigotes. The two last configurations confer high antigenic properties. In this review we discuss the different structures found and we pose the questions that still need investigation on the exchange of the configurations of galactose.
Keywords: Trypanosoma cruzi; mucins; α-galactopyranose; β-galactofuranose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Synthesis of a model trisaccharide for studying the interplay between the anti α-Gal antibody and the trans-sialidase reactions in Trypanosoma cruzi.Carbohydr Res. 2017 Oct 10;450:30-37. doi: 10.1016/j.carres.2017.08.007. Epub 2017 Aug 18. Carbohydr Res. 2017. PMID: 28858610
-
Synthesis of the O-linked hexasaccharide containing β-D-Galp-(1→2)-D-Galf in Trypanosoma cruzi mucins. Differences on sialylation by trans-sialidase of the two constituent hexasaccharides.Bioorg Med Chem. 2015 Mar 15;23(6):1213-22. doi: 10.1016/j.bmc.2015.01.056. Epub 2015 Feb 7. Bioorg Med Chem. 2015. PMID: 25703305
-
Synthesis of the hexasaccharide from Trypanosoma cruzi mucins with the Galp(1 → 2)Galf unit constructed with a superarmed thiogalactopyranosyl donor.Carbohydr Res. 2019 Aug 1;482:107734. doi: 10.1016/j.carres.2019.06.013. Epub 2019 Jun 25. Carbohydr Res. 2019. PMID: 31271957
-
Trans-sialidase and mucins of Trypanosoma cruzi: an important interplay for the parasite.Carbohydr Res. 2011 Sep 6;346(12):1389-93. doi: 10.1016/j.carres.2011.04.006. Epub 2011 Apr 8. Carbohydr Res. 2011. PMID: 21645882 Review.
-
Addition of α-O-GlcNAc to threonine residues define the post-translational modification of mucin-like molecules in Trypanosoma cruzi.Glycoconj J. 2013 Oct;30(7):659-66. doi: 10.1007/s10719-013-9469-7. Epub 2013 Feb 21. Glycoconj J. 2013. PMID: 23430107 Free PMC article. Review.
Cited by
-
The endoplasmic reticulum of trypanosomatids: An unrevealed road for chemotherapy.Front Cell Infect Microbiol. 2022 Nov 10;12:1057774. doi: 10.3389/fcimb.2022.1057774. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439218 Free PMC article. Review.
-
Trypanosoma cruzi pathogenicity involves virulence factor expression and upregulation of bioenergetic and biosynthetic pathways.Virulence. 2022 Dec;13(1):1827-1848. doi: 10.1080/21505594.2022.2132776. Virulence. 2022. PMID: 36284085 Free PMC article.
-
Don't Be Surprised When These Surprise You: Some Infrequently Studied Sphingoid Bases, Metabolites, and Factors That Should Be Kept in Mind During Sphingolipidomic Studies.Int J Mol Sci. 2025 Jan 14;26(2):650. doi: 10.3390/ijms26020650. Int J Mol Sci. 2025. PMID: 39859363 Free PMC article. Review.
-
The Potential Use of Peptides in the Fight against Chagas Disease and Leishmaniasis.Pharmaceutics. 2024 Feb 4;16(2):227. doi: 10.3390/pharmaceutics16020227. Pharmaceutics. 2024. PMID: 38399281 Free PMC article. Review.
-
Interaction With the Extracellular Matrix Triggers Calcium Signaling in Trypanosoma cruzi Prior to Cell Invasion.Front Cell Infect Microbiol. 2021 Oct 4;11:731372. doi: 10.3389/fcimb.2021.731372. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34671568 Free PMC article.
References
-
- Zingales B., Miles M.A., Campbell D.A., Tibayrenc M., Macedo A.M., Teixeira M.M., Schijman A.G., Llewellyn M.S., Lages-Silva E., Machado C.R., et al. The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

