Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic
- PMID: 32867241
- PMCID: PMC7559814
- DOI: 10.3390/pharmaceutics12090815
Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic
Abstract
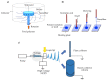
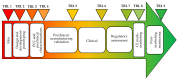
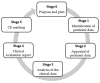
Chronic wounds, such as pressure ulcers, diabetic ulcers, venous ulcers and arterial insufficiency ulcers, are lesions that fail to proceed through the normal healing process within a period of 12 weeks. The treatment of skin chronic wounds still represents a great challenge. Wound medical devices (MDs) range from conventional and advanced dressings, up to skin grafts, but none of these are generally recognized as a gold standard. Based on recent developments, this paper reviews nanotechnology-based medical devices intended as skin substitutes. In particular, nanofibrous scaffolds are promising platforms for wound healing, especially due to their similarity to the extracellular matrix (ECM) and their capability to promote cell adhesion and proliferation, and to restore skin integrity, when grafted into the wound site. Nanotechnology-based scaffolds are emphasized here. The discussion will be focused on the definition of critical quality attributes (chemical and physical characterization, stability, particle size, surface properties, release of nanoparticles from MDs, sterility and apyrogenicity), the preclinical evaluation (biocompatibility testing, alternative in vitro tests for irritation and sensitization, wound healing test and animal wound models), the clinical evaluation and the CE (European Conformity) marking of nanotechnology-based MDs.
Keywords: CE marking; biocompatibility; chronic wounds; clinical evaluation; critical quality attributes; medical devices; nanotechnologies; wound models.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Engineered Biopolymeric Scaffolds for Chronic Wound Healing.Front Physiol. 2016 Aug 5;7:341. doi: 10.3389/fphys.2016.00341. eCollection 2016. Front Physiol. 2016. PMID: 27547189 Free PMC article. Review.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.Health Technol Assess. 2000;4(21):1-237. Health Technol Assess. 2000. PMID: 11074391 Review.
-
Poorly designed research does not help clarify the role of hyperbaric oxygen in the treatment of chronic diabetic foot ulcers.Diving Hyperb Med. 2016 Sep;46(3):133-134. Diving Hyperb Med. 2016. PMID: 27723012
-
Current Status and Future of Skin Substitutes for Chronic Wound Healing.J Cutan Med Surg. 2017 Jan/Feb;21(1):23-30. doi: 10.1177/1203475416664037. Epub 2016 Aug 20. J Cutan Med Surg. 2017. PMID: 27530398 Review.
-
An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings.Mini Rev Med Chem. 2018 Feb 14;18(5):414-427. doi: 10.2174/1389557517666170308112147. Mini Rev Med Chem. 2018. PMID: 28271816 Review.
Cited by
-
Promotive effects of four herbal medicine ARCC on wound healing in mice and human.Health Sci Rep. 2022 Apr 20;5(3):e494. doi: 10.1002/hsr2.494. eCollection 2022 May. Health Sci Rep. 2022. PMID: 35509387 Free PMC article.
-
Inorganic Nanomaterials in Tissue Engineering.Pharmaceutics. 2022 May 26;14(6):1127. doi: 10.3390/pharmaceutics14061127. Pharmaceutics. 2022. PMID: 35745700 Free PMC article. Review.
-
Experimental Study on Wound Area Measurement with Mobile Devices.Sensors (Basel). 2021 Aug 26;21(17):5762. doi: 10.3390/s21175762. Sensors (Basel). 2021. PMID: 34502653 Free PMC article.
-
Isolation of Aloe saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing.Pharmaceutics. 2022 Sep 8;14(9):1905. doi: 10.3390/pharmaceutics14091905. Pharmaceutics. 2022. PMID: 36145653 Free PMC article.
-
Nanotechnology development in surgical applications: recent trends and developments.Eur J Med Res. 2023 Nov 24;28(1):537. doi: 10.1186/s40001-023-01429-4. Eur J Med Res. 2023. PMID: 38001554 Free PMC article. Review.
References
-
- Mofazzal Jahromi M.A., Sahandi Zangabad P., Moosavi Basri S.M., Sahandi Zangabad K., Ghamarypour A., Aref A.R., Karimi M., Hamblin M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018;123:33–64. doi: 10.1016/j.addr.2017.08.001. - DOI - PMC - PubMed
-
- Garcia-Villen F., Faccendini A., Aguzzi C., Cerezo P., Bonferoni M.C., Rossi S., Grisoli P., Ruggeri M., Ferrari F., Sandri G., et al. Montmorillonite-norfloxacin nanocomposite intended for healing of infected wounds. Int. J. Nanomed. 2019;14:5051–5060. doi: 10.2147/IJN.S208713. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

