Aryl Hydrocarbon Receptor Role in Co-Ordinating SARS-CoV-2 Entry and Symptomatology: Linking Cytotoxicity Changes in COVID-19 and Cancers; Modulation by Racial Discrimination Stress
- PMID: 32867244
- PMCID: PMC7564943
- DOI: 10.3390/biology9090249
Aryl Hydrocarbon Receptor Role in Co-Ordinating SARS-CoV-2 Entry and Symptomatology: Linking Cytotoxicity Changes in COVID-19 and Cancers; Modulation by Racial Discrimination Stress
Abstract
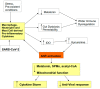
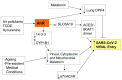
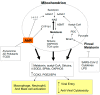
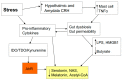
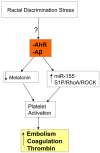
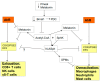
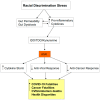
There is an under-recognized role of the aryl hydrocarbon receptor (AhR) in co-ordinating the entry and pathophysiology of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) that underpins the COVID-19 pandemic. The rise in pro-inflammatory cytokines during the 'cytokine storm' induce indoleamine 2,3-dioxygenase (IDO), leading to an increase in kynurenine that activates the AhR, thereby heightening the initial pro-inflammatory cytokine phase and suppressing the endogenous anti-viral response. Such AhR-driven changes underpin the heightened severity and fatality associated with pre-existent high-risk medical conditions, such as type II diabetes, as well as to how racial discrimination stress contributes to the raised severity/fatality in people from the Black Asian and Minority Ethnic (BAME) communities. The AhR is pivotal in modulating mitochondrial metabolism and co-ordinating specialized, pro-resolving mediators (SPMs), the melatonergic pathways, acetyl-coenzyme A, and the cyclooxygenase (COX) 2-prostaglandin (PG) E2 pathway that underpin 'exhaustion' in the endogenous anti-viral cells, paralleling similar metabolic suppression in cytolytic immune cells that is evident across all cancers. The pro-inflammatory cytokine induced gut permeability/dysbiosis and suppression of pineal melatonin are aspects of the wider pathophysiological underpinnings regulated by the AhR. This has a number of prophylactic and treatment implications for SARS-CoV-2 infection and cancers and future research directions that better investigate the biological underpinnings of social processes and how these may drive health disparities.
Keywords: COVID-19; SARS-CoV-2; acetyl-CoA; aryl hydrocarbon receptor; cancer; immune; melatonin; mitochondria; racism; treatment.
Conflict of interest statement
The authors declare that there are no conflict of interest with respect to the authorship and/or publication of this article.
Figures
References
-
- Anderson G., Reiter R.J. COVID-19 pathophysiology: Interactions of gut microbiome, melatonin, vitamin D, stress, kynurenine and the alpha 7 nicotinic receptor: Treatment implications. Melatonin Res. 2020;3:322–345. doi: 10.32794/mr11250066. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

