Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides
- PMID: 32867265
- PMCID: PMC7563103
- DOI: 10.3390/biom10091245
Enzymatic Regulation and Biological Functions of Reactive Cysteine Persulfides and Polysulfides
Abstract
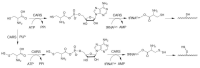
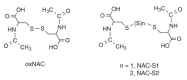
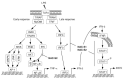
Cysteine persulfide (CysSSH) and cysteine polysulfides (CysSSnH, n > 1) are cysteine derivatives that have sulfane sulfur atoms bound to cysteine thiol. Advances in analytical methods that detect and quantify persulfides and polysulfides have shown that CysSSH and related species such as glutathione persulfide occur physiologically and are prevalent in prokaryotes, eukaryotes, and mammals in vivo. The chemical properties and abundance of these compounds suggest a central role for reactive persulfides in cell-regulatory processes. CysSSH and related species have been suggested to act as powerful antioxidants and cellular protectants and may serve as redox signaling intermediates. It was recently shown that cysteinyl-tRNA synthetase (CARS) is a new cysteine persulfide synthase. In addition, we discovered that CARS is involved in protein polysulfidation that is coupled with translation. Mitochondrial activity in biogenesis and bioenergetics is supported and upregulated by CysSSH derived from mitochondrial CARS. In this review article, we discuss the mechanisms of the biosynthesis of CysSSH and related persulfide species, with a particular focus on the roles of CARS. We also review the antioxidative and anti-inflammatory actions of persulfides.
Keywords: anti-inflammatory effect; antioxidant; cysteine persulfide; cysteinyl-tRNA synthetase; oxidative stress; sulfur respiration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Persulfide synthases that are functionally coupled with translation mediate sulfur respiration in mammalian cells.Br J Pharmacol. 2019 Feb;176(4):607-615. doi: 10.1111/bph.14356. Epub 2018 Jun 7. Br J Pharmacol. 2019. PMID: 29748969 Free PMC article. Review.
-
Antioxidative and anti-inflammatory actions of reactive cysteine persulfides.J Clin Biochem Nutr. 2021 Jan;68(1):5-8. doi: 10.3164/jcbn.20-13. Epub 2020 Jun 19. J Clin Biochem Nutr. 2021. PMID: 33536706 Free PMC article. Review.
-
Redox Signaling Regulated by Cysteine Persulfide and Protein Polysulfidation.Molecules. 2016 Dec 15;21(12):1721. doi: 10.3390/molecules21121721. Molecules. 2016. PMID: 27983699 Free PMC article. Review.
-
Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics.Nat Commun. 2017 Oct 27;8(1):1177. doi: 10.1038/s41467-017-01311-y. Nat Commun. 2017. PMID: 29079736 Free PMC article.
-
Methods in sulfide and persulfide research.Nitric Oxide. 2021 Nov 1;116:47-64. doi: 10.1016/j.niox.2021.09.002. Epub 2021 Sep 14. Nitric Oxide. 2021. PMID: 34534626 Free PMC article.
Cited by
-
Persulfidome of Sweet Pepper Fruits during Ripening: The Case Study of Leucine Aminopeptidase That Is Positively Modulated by H2S.Antioxidants (Basel). 2024 Jun 13;13(6):719. doi: 10.3390/antiox13060719. Antioxidants (Basel). 2024. PMID: 38929158 Free PMC article.
-
Structural and biochemical characterization of an encapsulin-associated rhodanese from Acinetobacter baumannii.Protein Sci. 2024 Aug;33(8):e5129. doi: 10.1002/pro.5129. Protein Sci. 2024. PMID: 39073218 Free PMC article.
-
Identification of Fungal Metabolite Gliotoxin as a Potent Inhibitor Against Bacterial O-Acetylserine Sulfhydrylase CysK and CysM.Int J Mol Sci. 2025 Jan 27;26(3):1106. doi: 10.3390/ijms26031106. Int J Mol Sci. 2025. PMID: 39940875 Free PMC article.
-
Cysteine-rich zinc finger proteins and the nuclear factor kappa-B pathway.Front Chem Biol. 2024;3:1503390. doi: 10.3389/fchbi.2024.1503390. Epub 2024 Dec 19. Front Chem Biol. 2024. PMID: 40405983 Free PMC article.
-
Differential Roles of Cystathionine Gamma-Lyase and Mercaptopyruvate Sulfurtransferase in Hapten-Induced Colitis and Contact Dermatitis in Mice.Int J Mol Sci. 2023 Jan 31;24(3):2659. doi: 10.3390/ijms24032659. Int J Mol Sci. 2023. PMID: 36768979 Free PMC article.
References
-
- Sawa T., Ono K., Tsutsuki H., Zhang T., Ida T., Nishida M., Akaike T. Reactive cysteine persulphides: Occurrence, biosynthesis, antioxidant activity, methodologies, and bacterial persulphide signalling. Adv. Microb. Physiol. 2018;72:1–28. - PubMed
-
- Fukuto J.M., Ignarro L.J., Nagy P., Wink D.A., Kevil C.G., Feelisch M., Cortese-Krott M.M., Bianco C.L., Kumagai Y., Hobbs A.J., et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018;592:2140–2152. doi: 10.1002/1873-3468.13090. - DOI - PMC - PubMed
-
- Motohashi H., Akaike T. Sulfur-utilizing cytoprotection and energy metabolism. Curr. Opin. Physiol. 2019;9:1–8. doi: 10.1016/j.cophys.2019.03.003. - DOI
-
- Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA. 2014;111:7606–7611. doi: 10.1073/pnas.1321232111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 18H02098/The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan/International
- 19K22258/The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan/International
- 18H02621/Ministry of Education, Culture, Sports, Science and Technology/International
- 16H04674/Ministry of Education, Culture, Sports, Science and Technology/International
- 18H05277/Ministry of Education, Culture, Sports, Science and Technology/International
LinkOut - more resources
Full Text Sources
Other Literature Sources

