Surface and Interface Designs in Copper-Based Conductive Inks for Printed/Flexible Electronics
- PMID: 32867267
- PMCID: PMC7559014
- DOI: 10.3390/nano10091689
Surface and Interface Designs in Copper-Based Conductive Inks for Printed/Flexible Electronics
Abstract
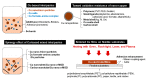
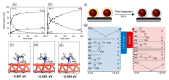
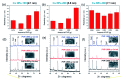
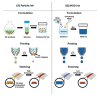
Silver (Ag), gold (Au), and copper (Cu) have been utilized as metals for fabricating metal-based inks/pastes for printed/flexible electronics. Among them, Cu is the most promising candidate for metal-based inks/pastes. Cu has high intrinsic electrical/thermal conductivity, which is more cost-effective and abundant, as compared to Ag. Moreover, the migration tendency of Cu is less than that of Ag. Thus, recently, Cu-based inks/pastes have gained increasing attention as conductive inks/pastes for printed/flexible electronics. However, the disadvantages of Cu-based inks/pastes are their instability against oxidation under an ambient condition and tendency to form insulating layers of Cu oxide, such as cuprous oxide (Cu2O) and cupric oxide (CuO). The formation of the Cu oxidation causes a low conductivity in sintered Cu films and interferes with the sintering of Cu particles. In this review, we summarize the surface and interface designs for Cu-based conductive inks/pastes, in which the strategies for the oxidation resistance of Cu and low-temperature sintering are applied to produce highly conductive Cu patterns/electrodes on flexible substrates. First, we classify the Cu-based inks/pastes and briefly describe the surface oxidation behaviors of Cu. Next, we describe various surface control approaches for Cu-based inks/pastes to achieve both the oxidation resistance and low-temperature sintering to produce highly conductive Cu patterns/electrodes on flexible substrates. These surface control approaches include surface designs by polymers, small ligands, core-shell structures, and surface activation. Recently developed Cu-based mixed inks/pastes are also described, and the synergy effect in the mixed inks/pastes offers improved performances compared with the single use of each component. Finally, we offer our perspectives on Cu-based inks/pastes for future efforts.
Keywords: complexes; copper; flexible devices; inks; nanoparticles; pastes; printed electronics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















Similar articles
-
Fabrication of Conductive Copper Films on Flexible Polymer Substrates by Low-Temperature Sintering of Composite Cu Ink in Air.ACS Appl Mater Interfaces. 2017 Jun 21;9(24):20852-20858. doi: 10.1021/acsami.7b04641. Epub 2017 Jun 8. ACS Appl Mater Interfaces. 2017. PMID: 28574247
-
Self-Organizing, Environmentally Stable, and Low-Cost Copper-Nickel Complex Inks for Printed Flexible Electronics.ACS Appl Mater Interfaces. 2022 Feb 16;14(6):8146-8156. doi: 10.1021/acsami.1c21633. Epub 2022 Feb 1. ACS Appl Mater Interfaces. 2022. PMID: 35104116
-
Low-Thermal-Budget Photonic Processing of Highly Conductive Cu Interconnects Based on CuO Nanoinks: Potential for Flexible Printed Electronics.ACS Appl Mater Interfaces. 2016 Jan 27;8(3):2441-8. doi: 10.1021/acsami.5b12156. Epub 2016 Jan 12. ACS Appl Mater Interfaces. 2016. PMID: 26720684
-
Copper inks for printed electronics: a review.Nanoscale. 2022 Nov 10;14(43):16003-16032. doi: 10.1039/d2nr03990g. Nanoscale. 2022. PMID: 36301077 Review.
-
Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics.Int J Mol Sci. 2019 Apr 29;20(9):2124. doi: 10.3390/ijms20092124. Int J Mol Sci. 2019. PMID: 31036787 Free PMC article. Review.
Cited by
-
Electrochemical Deposition and Etching of Quasi-Two-Dimensional Periodic Membrane Structure.Molecules. 2024 Apr 13;29(8):1775. doi: 10.3390/molecules29081775. Molecules. 2024. PMID: 38675596 Free PMC article.
-
Effect of Copper Surface Roughness on the High-Temperature Structural Stability of Single-Layer-Graphene.Materials (Basel). 2024 Apr 3;17(7):1648. doi: 10.3390/ma17071648. Materials (Basel). 2024. PMID: 38612164 Free PMC article.
-
UV-Vis Sintering Process for Fabrication of Conductive Coatings Based on Ni-Ag Core-Shell Nanoparticles.Materials (Basel). 2023 Nov 17;16(22):7218. doi: 10.3390/ma16227218. Materials (Basel). 2023. PMID: 38005147 Free PMC article.
-
Facile Preparation of Monodisperse Cu@Ag Core-Shell Nanoparticles for Conductive Ink in Printing Electronics.Micromachines (Basel). 2023 Jun 27;14(7):1318. doi: 10.3390/mi14071318. Micromachines (Basel). 2023. PMID: 37512629 Free PMC article.
-
Rapid Detection of Cleanliness on Direct Bonded Copper Substrate by Using UV Hyperspectral Imaging.Sensors (Basel). 2024 Jul 19;24(14):4680. doi: 10.3390/s24144680. Sensors (Basel). 2024. PMID: 39066077 Free PMC article.
References
-
- Davis J.R. Asm Specialty Handbook: Copper and Copper Alloys. ASM International; Novelty, OH, USA: 2001.
-
- Sreenilayam S.P., Ahad I.U., Nicolosi V., Acinas Garzon V., Brabazon D. Advanced Materials of Printed Wearables for Physiological Parameter Monitoring. Mater. Today. 2020;32:147–177. doi: 10.1016/j.mattod.2019.08.005. - DOI
-
- Fernandes D.F., Majidi C., Tavakoli M. Digitally Printed Stretchable Electronics: A Review. J. Mater. Chem. C. 2019;7:14035–14068. doi: 10.1039/C9TC04246F. - DOI
-
- Huang Y., Fan X., Chen S.-C., Zhao N. Emerging Technologies of Flexible Pressure Sensors: Materials, Modeling, Devices, and Manufacturing. Adv. Funct. Mater. 2019;29:1808509. doi: 10.1002/adfm.201808509. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

