Dynamic Nuclear Polarization of Biomembrane Assemblies
- PMID: 32867275
- PMCID: PMC7565305
- DOI: 10.3390/biom10091246
Dynamic Nuclear Polarization of Biomembrane Assemblies
Abstract
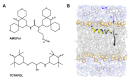
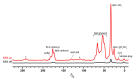
While atomic scale structural and dynamic information are hallmarks of nuclear magnetic resonance (NMR) methodologies, sensitivity is a fundamental limitation in NMR studies. Fully exploiting NMR capabilities to study membrane proteins is further hampered by their dilution within biological membranes. Recent developments in dynamic nuclear polarization (DNP), which can transfer the relatively high polarization of unpaired electrons to nuclear spins, show promise for overcoming the sensitivity bottleneck and enabling NMR characterization of membrane proteins under native-like conditions. Here we discuss fundamental aspects of DNP-enhanced solid-state NMR spectroscopy, experimental details relevant to the study of lipid assemblies and incorporated proteins, and sensitivity gains which can be realized in biomembrane-based samples. We also present unique insights which can be gained from DNP measurements and prospects for further development of the technique for elucidating structures and orientations of membrane proteins in native lipid environments.
Keywords: Dynamic Nuclear Polarization (DNP); Nuclear Magnetic Resonance (NMR); membrane active peptides; membrane proteins; solid-state nuclear magnetic resonance (ssNMR).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Dynamic nuclear polarization methods in solids and solutions to explore membrane proteins and membrane systems.Annu Rev Phys Chem. 2013;64:507-32. doi: 10.1146/annurev-physchem-040412-110028. Epub 2013 Jan 16. Annu Rev Phys Chem. 2013. PMID: 23331309 Review.
-
DNP NMR of biomolecular assemblies.J Struct Biol. 2019 Apr 1;206(1):90-98. doi: 10.1016/j.jsb.2018.09.011. Epub 2018 Sep 29. J Struct Biol. 2019. PMID: 30273657 Review.
-
Dynamic Nuclear Polarization Illuminates Key Protein-Lipid Interactions in the Native Bacterial Cell Envelope.Biochemistry. 2023 Aug 1;62(15):2252-2256. doi: 10.1021/acs.biochem.3c00262. Epub 2023 Jul 17. Biochemistry. 2023. PMID: 37459255 Free PMC article.
-
Efficient DNP NMR of membrane proteins: sample preparation protocols, sensitivity, and radical location.J Biomol NMR. 2016 Mar;64(3):223-37. doi: 10.1007/s10858-016-0023-3. Epub 2016 Feb 12. J Biomol NMR. 2016. PMID: 26873390 Free PMC article.
-
High-sensitivity protein solid-state NMR spectroscopy.Curr Opin Struct Biol. 2019 Oct;58:183-190. doi: 10.1016/j.sbi.2019.03.027. Epub 2019 Apr 25. Curr Opin Struct Biol. 2019. PMID: 31031067 Free PMC article. Review.
Cited by
-
Resolution in cryogenic solid state NMR: Challenges and solutions.Protein Sci. 2024 Jul;33(7):e4803. doi: 10.1002/pro.4803. Protein Sci. 2024. PMID: 37847566 Free PMC article. Review.
-
Water Accessibility Refinement of the Extended Structure of KirBac1.1 in the Closed State.Front Mol Biosci. 2021 Nov 30;8:772855. doi: 10.3389/fmolb.2021.772855. eCollection 2021. Front Mol Biosci. 2021. PMID: 34917650 Free PMC article.
-
In-Cell Sensitivity-Enhanced NMR of Intact Viable Mammalian Cells.J Am Chem Soc. 2021 Nov 10;143(44):18454-18466. doi: 10.1021/jacs.1c06680. Epub 2021 Nov 1. J Am Chem Soc. 2021. PMID: 34724614 Free PMC article.
-
Fine optimization of a dissolution dynamic nuclear polarization experimental setting for 13C NMR of metabolic samples.Magn Reson (Gott). 2022 Sep 29;3(2):183-202. doi: 10.5194/mr-3-183-2022. eCollection 2022. Magn Reson (Gott). 2022. PMID: 37904870 Free PMC article.
-
DNP-assisted solid-state NMR enables detection of proteins at nanomolar concentrations in fully protonated cellular milieu.J Biomol NMR. 2024 Jun;78(2):95-108. doi: 10.1007/s10858-024-00436-9. Epub 2024 Mar 23. J Biomol NMR. 2024. PMID: 38520488 Free PMC article.
References
-
- Amani R., Borcik C.G., Khan N.H., Versteeg D.B., Yekefallah M., Do H.Q., Coats H.R., Wylie B.J. Conformational Changes upon Gating of KirBac1.1 into an Open-Activated State Revealed by Solid-State NMR and Functional Assays. Proc. Natl. Acad. Sci. USA. 2020;117:2938–2947. doi: 10.1073/pnas.1915010117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

