Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization
- PMID: 32867356
- PMCID: PMC7504408
- DOI: 10.3390/molecules25173923
Evaluation of Supercritical CO2-Assisted Protocols in a Model of Ovine Aortic Root Decellularization
Abstract
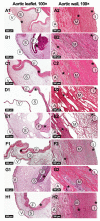
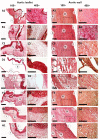
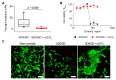
One of the leading trends in the modern tissue engineering is the development of new effective methods of decellularization aimed at the removal of cellular components from a donor tissue, reducing its immunogenicity and the risk of rejection. Supercritical CO2 (scCO2)-assisted processing has been proposed to improve the outcome of decellularization, reduce contamination and time costs. The resulting products can serve as personalized tools for tissue-engineering therapy of various somatic pathologies. However, the decellularization of heterogeneous 3D structures, such as the aortic root, requires optimization of the parameters, including preconditioning medium composition, the type of co-solvent, values of pressure and temperature inside the scCO2 reactor, etc. In our work, using an ovine aortic root model, we performed a comparative analysis of the effectiveness of decellularization approaches based on various combinations of these parameters. The protocols were based on the combinations of treatments in alkaline, ethanol or detergent solutions with scCO2-assisted processing at different modes. Histological analysis demonstrated favorable effects of the preconditioning in a detergent solution. Following processing in scCO2 medium provided a high decellularization degree, reduced cytotoxicity, and increased ultimate tensile strength and Young's modulus of the aortic valve leaflets, while the integrity of the extracellular matrix was preserved.
Keywords: aortic valve; biomaterials; biomedical engineering; decellularization; supercritical CO2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Use of supercritical CO2 in soft tissue decellularization.Methods Cell Biol. 2020;157:49-79. doi: 10.1016/bs.mcb.2019.10.012. Epub 2019 Dec 10. Methods Cell Biol. 2020. PMID: 32334720
-
Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis.Acta Biomater. 2018 Feb;67:270-281. doi: 10.1016/j.actbio.2017.11.046. Epub 2017 Dec 6. Acta Biomater. 2018. PMID: 29223704
-
Supercritical Carbon Dioxide-Assisted Decellularization of Aorta and Cornea.Tissue Eng Part C Methods. 2017 Sep;23(9):540-547. doi: 10.1089/ten.TEC.2017.0090. Epub 2017 Aug 17. Tissue Eng Part C Methods. 2017. PMID: 28726559
-
Contributions of supercritical fluid technology for advancing decellularization and postprocessing of viable biological materials.Mater Horiz. 2022 Mar 7;9(3):864-891. doi: 10.1039/d1mh01720a. Mater Horiz. 2022. PMID: 34931632 Review.
-
Supercritical Fluid-Based Decellularization Technologies for Regenerative Medicine Applications.Macromol Biosci. 2021 Aug;21(8):e2100160. doi: 10.1002/mabi.202100160. Epub 2021 Jun 13. Macromol Biosci. 2021. PMID: 34121330 Review.
Cited by
-
Advances and Challenges of Tissue Vascular Scaffolds and Supercritical Carbon Dioxide Technology in Cardiovascular Diseases.Tissue Eng Regen Med. 2025 Apr;22(3):273-284. doi: 10.1007/s13770-025-00710-3. Epub 2025 Mar 3. Tissue Eng Regen Med. 2025. PMID: 40029563 Review.
-
Moisture adsorption by decellularized bovine pericardium collagen matrices studied by terahertz pulsed spectroscopy and solid immersion microscopy.Biomed Opt Express. 2021 Aug 2;12(9):5368-5386. doi: 10.1364/BOE.433216. eCollection 2021 Sep 1. Biomed Opt Express. 2021. PMID: 34692188 Free PMC article.
-
Decellularized extracellular matrix mediates tissue construction and regeneration.Front Med. 2022 Feb;16(1):56-82. doi: 10.1007/s11684-021-0900-3. Epub 2021 Dec 28. Front Med. 2022. PMID: 34962624 Free PMC article. Review.
-
Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges.Int J Mol Sci. 2022 Oct 27;23(21):13040. doi: 10.3390/ijms232113040. Int J Mol Sci. 2022. PMID: 36361824 Free PMC article. Review.
-
Preparation and characterization of human decellularized ovarian scaffold based on supercritical carbon dioxide protocol.Biomed Eng Online. 2025 May 13;24(1):59. doi: 10.1186/s12938-025-01392-7. Biomed Eng Online. 2025. PMID: 40361140 Free PMC article.
References
-
- Sedrakyan A., Hebert P., Vaccarino V., Paltiel A.D., Elefteriades J.A., Mattera J., Lin Z., Roumanis S.A., Krumholz H.M. Quality of life after aortic valve replacement with tissue and mechanical implants. J. Thorac. Cardiovasc. Surg. 2004;128:266–272. doi: 10.1016/j.jtcvs.2003.12.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

