Nuclear Receptors as Autophagy-Based Antimicrobial Therapeutics
- PMID: 32867365
- PMCID: PMC7563212
- DOI: 10.3390/cells9091979
Nuclear Receptors as Autophagy-Based Antimicrobial Therapeutics
Abstract
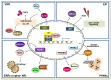
Autophagy is an intracellular process that targets intracellular pathogens for lysosomal degradation. Autophagy is tightly controlled at transcriptional and post-translational levels. Nuclear receptors (NRs) are a family of transcriptional factors that regulate the expression of gene sets involved in, for example, metabolic and immune homeostasis. Several NRs show promise as host-directed anti-infectives through the modulation of autophagy activities by their natural ligands or small molecules (agonists/antagonists). Here, we review the roles and mechanisms of NRs (vitamin D receptors, estrogen receptors, estrogen-related receptors, and peroxisome proliferator-activated receptors) in linking immunity and autophagy during infection. We also discuss the potential of emerging NRs (REV-ERBs, retinoic acid receptors, retinoic acid-related orphan receptors, liver X receptors, farnesoid X receptors, and thyroid hormone receptors) as candidate antimicrobials. The identification of novel roles and mechanisms for NRs will enable the development of autophagy-adjunctive therapeutics for emerging and re-emerging infectious diseases.
Keywords: autophagy; host defense; infections; nuclear receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Transcriptional Regulation of Hepatic Autophagy by Nuclear Receptors.Cells. 2022 Feb 10;11(4):620. doi: 10.3390/cells11040620. Cells. 2022. PMID: 35203271 Free PMC article. Review.
-
Macrophage nuclear receptors: Emerging key players in infectious diseases.PLoS Pathog. 2019 Mar 21;15(3):e1007585. doi: 10.1371/journal.ppat.1007585. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30897154 Free PMC article. Review.
-
Role of Class II nuclear receptors in liver carcinogenesis.Anticancer Agents Med Chem. 2011 Jul;11(6):529-42. doi: 10.2174/187152011796011064. Anticancer Agents Med Chem. 2011. PMID: 21554208 Review.
-
Autophagy: A new strategy for host-directed therapy of tuberculosis.Virulence. 2019 Dec;10(1):448-459. doi: 10.1080/21505594.2018.1536598. Epub 2018 Nov 2. Virulence. 2019. PMID: 30322337 Free PMC article. Review.
-
Application of Machine Learning Methods in Predicting Nuclear Receptors and their Families.Med Chem. 2020;16(5):594-604. doi: 10.2174/1573406415666191004125551. Med Chem. 2020. PMID: 31584374 Review.
Cited by
-
Antibiotic Therapy of Plague: A Review.Biomolecules. 2021 May 12;11(5):724. doi: 10.3390/biom11050724. Biomolecules. 2021. PMID: 34065940 Free PMC article. Review.
-
Nuclear Receptors as Multiple Regulators of NLRP3 Inflammasome Function.Front Immunol. 2021 Feb 26;12:630569. doi: 10.3389/fimmu.2021.630569. eCollection 2021. Front Immunol. 2021. PMID: 33717162 Free PMC article. Review.
-
Archaic connectivity between the sulfated heparan sulfate and the herpesviruses - An evolutionary potential for cross-species interactions.Comput Struct Biotechnol J. 2023 Jan 13;21:1030-1040. doi: 10.1016/j.csbj.2023.01.005. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 36733705 Free PMC article. Review.
-
Transcriptional Regulation of Hepatic Autophagy by Nuclear Receptors.Cells. 2022 Feb 10;11(4):620. doi: 10.3390/cells11040620. Cells. 2022. PMID: 35203271 Free PMC article. Review.
-
Basal Autophagy Is Necessary for A Pharmacologic PPARα Transactivation.Cells. 2022 Feb 21;11(4):754. doi: 10.3390/cells11040754. Cells. 2022. PMID: 35203398 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

