A Novel Peptide Antibiotic, Pro10-1D, Designed from Insect Defensin Shows Antibacterial and Anti-Inflammatory Activities in Sepsis Models
- PMID: 32867384
- PMCID: PMC7504360
- DOI: 10.3390/ijms21176216
A Novel Peptide Antibiotic, Pro10-1D, Designed from Insect Defensin Shows Antibacterial and Anti-Inflammatory Activities in Sepsis Models
Abstract
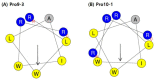
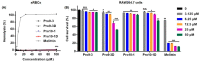
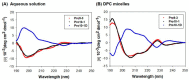
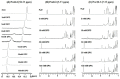
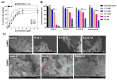
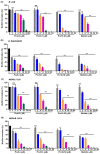
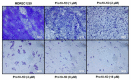
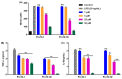
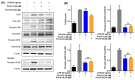
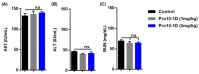
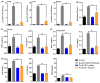
Owing to the challenges faced by conventional therapeutics, novel peptide antibiotics against multidrug-resistant (MDR) gram-negative bacteria need to be urgently developed. We had previously designed Pro9-3 and Pro9-3D from the defensin of beetle Protaetia brevitarsis; they showed high antimicrobial activity with cytotoxicity. Here, we aimed to develop peptide antibiotics with bacterial cell selectivity and potent antibacterial activity against gram-negative bacteria. We designed 10-meric peptides with increased cationicity by adding Arg to the N-terminus of Pro9-3 (Pro10-1) and its D-enantiomeric alteration (Pro10-1D). Among all tested peptides, the newly designed Pro10-1D showed the strongest antibacterial activity against Escherichia coli, Acinetobacter baumannii, and MDR strains with resistance against protease digestion. Pro10-1D can act as a novel potent peptide antibiotic owing to its outstanding inhibitory activities against bacterial film formation with high bacterial cell selectivity. Dye leakage and scanning electron microscopy revealed that Pro10-1D targets the bacterial membrane. Pro10-1D inhibited inflammation via Toll Like Receptor 4 (TLR4)/Nuclear factor-κB (NF-κB) signaling pathways in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Furthermore, Pro10-1D ameliorated multiple-organ damage and attenuated systemic infection-associated inflammation in an E. coli K1-induced sepsis mouse model. Overall, our results suggest that Pro10-1D can potentially serve as a novel peptide antibiotic for the treatment of gram-negative sepsis.
Keywords: antimicrobial peptide; biofilm; gram-negative infection; peptide antibiotics; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
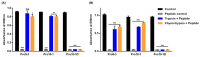

Similar articles
-
Anti-Endotoxin 9-Meric Peptide with Therapeutic Potential for the Treatment of Endotoxemia.J Microbiol Biotechnol. 2021 Jan 28;31(1):25-32. doi: 10.4014/jmb.2011.11011. J Microbiol Biotechnol. 2021. PMID: 33263333 Free PMC article.
-
Antiseptic 9-Meric Peptide with Potency against Carbapenem-Resistant Acinetobacter baumannii Infection.Int J Mol Sci. 2021 Nov 20;22(22):12520. doi: 10.3390/ijms222212520. Int J Mol Sci. 2021. PMID: 34830401 Free PMC article.
-
Roles of the Conserved Amino Acid Residues in Reduced Human Defensin 5: Cysteine and Arginine Are Indispensable for Its Antibacterial Action and LPS Neutralization.ChemMedChem. 2019 Aug 6;14(15):1457-1465. doi: 10.1002/cmdc.201900282. Epub 2019 Jul 10. ChemMedChem. 2019. PMID: 31290614
-
Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis.Biochim Biophys Acta. 2016 May;1858(5):971-9. doi: 10.1016/j.bbamem.2016.01.011. Epub 2016 Jan 20. Biochim Biophys Acta. 2016. PMID: 26801369 Review.
-
An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes.Biochim Biophys Acta. 2016 May;1858(5):958-70. doi: 10.1016/j.bbamem.2015.10.011. Epub 2015 Oct 21. Biochim Biophys Acta. 2016. PMID: 26498397 Review.
Cited by
-
Protective Effects of Rhamnetin in Carbapenem-Resistant Acinetobacter baumannii-Induced Sepsis Model and the Underlying Mechanism.Int J Mol Sci. 2023 Oct 26;24(21):15603. doi: 10.3390/ijms242115603. Int J Mol Sci. 2023. PMID: 37958587 Free PMC article.
-
Cationic antimicrobial peptide CC34 potential anticancer and apoptotic induction on cancer cells.Amino Acids. 2025 May 24;57(1):28. doi: 10.1007/s00726-025-03458-1. Amino Acids. 2025. PMID: 40413361 Free PMC article.
-
Microflora for improving the Auricularia auricula spent mushroom substrate for Protaetia brevitarsis production.iScience. 2022 Oct 7;25(11):105307. doi: 10.1016/j.isci.2022.105307. eCollection 2022 Nov 18. iScience. 2022. PMID: 36300006 Free PMC article.
-
Resistance, Tolerance, Virulence and Bacterial Pathogen Fitness-Current State and Envisioned Solutions for the Near Future.Pathogens. 2023 May 22;12(5):746. doi: 10.3390/pathogens12050746. Pathogens. 2023. PMID: 37242416 Free PMC article. Review.
-
Bioactive Peptides from Edible Mushrooms-The Preparation, Mechanisms, Structure-Activity Relationships and Prospects.Foods. 2023 Aug 2;12(15):2935. doi: 10.3390/foods12152935. Foods. 2023. PMID: 37569204 Free PMC article. Review.
References
-
- Tucureanu M.M., Rebleanu D., Constantinescu C.A., Deleanu M., Voicu G., Butoi E., Calin M., Manduteanu I. Lipopolysaccharide-induced inflammation in monocytes/macrophages is blocked by liposomal delivery of Gi-protein inhibitor. Int. J. Nanomed. 2017;13:63–76. doi: 10.2147/IJN.S150918. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

