Mesoporous Silica Nanoparticles in Bioimaging
- PMID: 32867401
- PMCID: PMC7504327
- DOI: 10.3390/ma13173795
Mesoporous Silica Nanoparticles in Bioimaging
Abstract
A biomedical contrast agent serves to enhance the visualisation of a specific (potentially targeted) physiological region. In recent years, mesoporous silica nanoparticles (MSNs) have developed as a flexible imaging platform of tuneable size/morphology, abundant surface chemistry, biocompatibility and otherwise useful physiochemical properties. This review discusses MSN structural types and synthetic strategies, as well as methods for surface functionalisation. Recent applications in biomedical imaging are then discussed, with a specific emphasis on magnetic resonance and optical modes together with utility in multimodal imaging.
Keywords: bioimaging; imaging modality; magnetic resonance imaging; mesoporous silica nanoparticles; multi-modality imaging; nanoparticles; optical imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
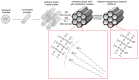

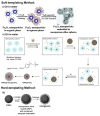




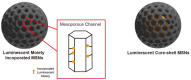



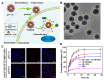



References
-
- Kresge C.T., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. doi: 10.1038/359710a0. - DOI
-
- Beck J.S., Vartuli J.C., Roth W.J., Leonowicz M.E., Kresge C.T., Schmitt K.D., Chu C.T.W., Olson D.H., Sheppard E.W., McCullen S.B., et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992;114:10834–10843. doi: 10.1021/ja00053a020. - DOI
-
- Hou L., Zheng Y., Wang Y., Hu Y., Shi J., Liu Q., Zhang H., Zhang Z. Self-Regulated Carboxyphenylboronic Acid-Modified Mesoporous Silica Nanoparticles with “Touch Switch” Releasing Property for Insulin Delivery. ACS Appl. Mater. Interfaces. 2018;10:21927–21938. doi: 10.1021/acsami.8b06998. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

