Biomaterials for Bioprinting Microvasculature
- PMID: 32867470
- PMCID: PMC7810139
- DOI: 10.1021/acs.chemrev.0c00027
Biomaterials for Bioprinting Microvasculature
Abstract
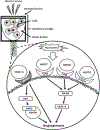
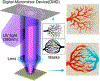
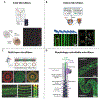
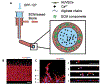
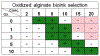
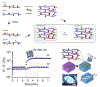
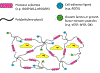
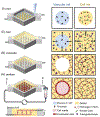
Microvasculature functions at the tissue and cell level, regulating local mass exchange of oxygen and nutrient-rich blood. While there has been considerable success in the biofabrication of large- and small-vessel replacements, functional microvasculature has been particularly challenging to engineer due to its size and complexity. Recently, three-dimensional bioprinting has expanded the possibilities of fabricating sophisticated microvascular systems by enabling precise spatiotemporal placement of cells and biomaterials based on computer-aided design. However, there are still significant challenges facing the development of printable biomaterials that promote robust formation and controlled 3D organization of microvascular networks. This review provides a thorough examination and critical evaluation of contemporary biomaterials and their specific roles in bioprinting microvasculature. We first provide an overview of bioprinting methods and techniques that enable the fabrication of microvessels. We then offer an in-depth critical analysis on the use of hydrogel bioinks for printing microvascularized constructs within the framework of current bioprinting modalities. We end with a review of recent applications of bioprinted microvasculature for disease modeling, drug testing, and tissue engineering, and conclude with an outlook on the challenges facing the evolution of biomaterials design for bioprinting microvasculature with physiological complexity.
Figures






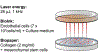

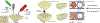









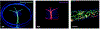








Similar articles
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
Converging functionality: Strategies for 3D hybrid-construct biofabrication and the role of composite biomaterials for skeletal regeneration.Acta Biomater. 2021 Sep 15;132:188-216. doi: 10.1016/j.actbio.2021.03.008. Epub 2021 Mar 10. Acta Biomater. 2021. PMID: 33713862 Review.
-
Recent trends in embedded 3D bioprinting of vascularized tissue constructs.Biofabrication. 2025 Feb 10;17(2). doi: 10.1088/1758-5090/adafdd. Biofabrication. 2025. PMID: 39879658 Review.
-
Bioprinted microvasculature: progressing from structure to function.Biofabrication. 2022 Feb 23;14(2):10.1088/1758-5090/ac4fb5. doi: 10.1088/1758-5090/ac4fb5. Biofabrication. 2022. PMID: 35086069 Free PMC article. Review.
-
Biomaterials in bone and mineralized tissue engineering using 3D printing and bioprinting technologies.Biomed Phys Eng Express. 2021 Oct 7;7(6). doi: 10.1088/2057-1976/ac21ab. Biomed Phys Eng Express. 2021. PMID: 34438382 Review.
Cited by
-
Handheld bioprinting strategies for in situ wound dressing.Essays Biochem. 2021 Aug 10;65(3):533-543. doi: 10.1042/EBC20200098. Essays Biochem. 2021. PMID: 34028545 Free PMC article. Review.
-
Blood vessels in a dish: the evolution, challenges, and potential of vascularized tissues and organoids.Front Cardiovasc Med. 2024 Jun 13;11:1336910. doi: 10.3389/fcvm.2024.1336910. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38938652 Free PMC article. Review.
-
In Situ 3D Bioprinting Living Photosynthetic Scaffolds for Autotrophic Wound Healing.Research (Wash D C). 2022 Mar 20;2022:9794745. doi: 10.34133/2022/9794745. eCollection 2022. Research (Wash D C). 2022. PMID: 35387266 Free PMC article.
-
Tissue-Penetrating Ultrasound-Triggered Hydrogel for Promoting Microvascular Network Reconstruction.Adv Sci (Weinh). 2024 Jun;11(23):e2401368. doi: 10.1002/advs.202401368. Epub 2024 Apr 10. Adv Sci (Weinh). 2024. PMID: 38600702 Free PMC article.
-
Accelerating Patterned Vascularization Using Granular Hydrogel Scaffolds and Surgical Micropuncture.Small. 2024 Feb;20(8):e2307928. doi: 10.1002/smll.202307928. Epub 2023 Oct 12. Small. 2024. PMID: 37824280 Free PMC article.
References
-
- Sherwood L. Human Physiology: From Cells to Systems; Cengage learning, 2015.
-
- Fox SI Human Physiology 9th Editon; McGraw-Hill press, New York, USA, 2006.
-
- Barral J-P; Croibier A. In Visceral Vascular Manipulations; Barral J-P;Croibier A, Eds.; Churchill Livingstone: Oxford, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

