A serological survey of SARS-CoV-2 in cat in Wuhan
- PMID: 32867625
- PMCID: PMC7534315
- DOI: 10.1080/22221751.2020.1817796
A serological survey of SARS-CoV-2 in cat in Wuhan
Abstract
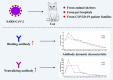
COVID-19 is a new respiratory illness caused by SARS-CoV-2, and has constituted a global public health emergency. Cat is susceptible to SARS-CoV-2. However, the prevalence of SARS-CoV-2 in cats remains largely unknown. Here, we investigated the infection of SARS-CoV-2 in cats during COVID-19 outbreak in Wuhan by serological detection methods. A cohort of serum samples were collected from cats in Wuhan, including 102 sampled after COVID-19 outbreak, and 39 prior to the outbreak. Fifteen sera collected after the outbreak were positive for the receptor binding domain (RBD) of SARS-CoV-2 by indirect enzyme linked immunosorbent assay (ELISA). Among them, 11 had SARS-CoV-2 neutralizing antibodies with a titer ranging from 1/20 to 1/1080. No serological cross-reactivity was detected between SARS-CoV-2 and type I or II feline infectious peritonitis virus (FIPV). In addition, we continuously monitored serum antibody dynamics of two positive cats every 10 days over 130 days. Their serum antibodies reached the peak at 10 days after first sampling, and declined to the limit of detection within 110 days. Our data demonstrated that SARS-CoV-2 has infected cats in Wuhan during the outbreak and described serum antibody dynamics in cats, providing an important reference for clinical treatment and prevention of COVID-19.
Keywords: COVID-19; SARS-CoV-2; cats; serological investigation; serum antibody dynamic.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
