The effect of maternal poliovirus antibodies on the immune responses of infants to poliovirus vaccines
- PMID: 32867698
- PMCID: PMC7460787
- DOI: 10.1186/s12879-020-05348-1
The effect of maternal poliovirus antibodies on the immune responses of infants to poliovirus vaccines
Abstract
Background: Maternal poliovirus antibodies could provide passive immunity to the newborns from poliovirus infection during their first few months of life, but they may impair the immune responses of infants to the poliovirus vaccine as well. In our study, we pooled the data from three clinical trials of the inactivated poliovirus vaccine (IPV) based on Sabin strains to investigate the effect of maternal poliovirus antibodies on the immune responses of infants to poliovirus vaccines.
Methods: There were five groups in the pooled analysis, including low-dose Sabin IPV, medium-dose Sabin IPV, high-dose Sabin IPV, control Sabin IPV, and control Salk IPV groups. We reclassified the infants in different groups according to their maternal poliovirus antibodies by two methods, the first one included maternal antibody negative (< 1:8) and maternal antibody positive (≥1:8), and the second one included maternal antibody titer < 1:8, 1:8 ~ < 1:32 and ≥ 1:32. Then, we compared the geometric mean titers (GMTs), geometric mean antibody fold increases (GMIs) and seroconversion rates of poliovirus type-specific neutralizing antibodies after vaccination among participants with different maternal poliovirus antibody levels.
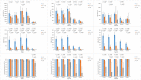
Results: The GMTs and GMIs of three types of poliovirus antibodies after vaccination in maternal antibody negative participants were significantly higher than those in maternal antibody positive participants. The seroconversion rates of type II and type III poliovirus antibodies in maternal antibody positive participants were significantly lower than those in maternal antibody negative participants. Among participants with maternal antibody titer < 1:8, 1:8 ~ < 1:32 and ≥ 1:32, the GMTs and GMIs of three types of poliovirus antibodies after vaccination showed a tendency to decline with the increasing of maternal antibody levels. The seroconversion rates of three types of poliovirus antibodies in participants with maternal antibody titer ≥1:32 were significantly lower than those in participants with maternal antibody titer < 1:8 and 1:8 ~ < 1:32.
Conclusions: Maternal poliovirus antibodies interfered with the immune responses of infants to poliovirus vaccines, and a high level of maternal antibodies exhibited a greater dampening effect.
Trial registration: ClinicalTrials.gov NCT04264598 February 11, 2020; ClinicalTrials.gov NCT04264546 February 11, 2020; ClinicalTrials.gov NCT03902054 April 3, 2019. Retrospectively registered.
Keywords: Immune response; Maternal antibody; Poliovirus vaccine.
Conflict of interest statement
Guifan Li is an employee of Beijing Minhai Biotechnology Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
Similar articles
-
Effect of maternal antibody on the infant immune response to inactivated poliovirus vaccines made from Sabin strains.Hum Vaccin Immunother. 2019;15(5):1160-1166. doi: 10.1080/21645515.2019.1572410. Epub 2019 Mar 19. Hum Vaccin Immunother. 2019. PMID: 30676838 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of sabin-strain based inactivated poliovirus vaccine replacing salk-strain based inactivated poliovirus vaccine: An innovative application of different strain-IPVs replacement.Vaccine. 2021 Apr 22;39(17):2467-2474. doi: 10.1016/j.vaccine.2021.02.073. Epub 2021 Mar 31. Vaccine. 2021. PMID: 33810904 Clinical Trial.
-
Immunogenicity of three sequential schedules with Sabin inactivated poliovirus vaccine and bivalent oral poliovirus vaccine in Zhejiang, China: an open-label, randomised, controlled trial.Lancet Infect Dis. 2020 Sep;20(9):1071-1079. doi: 10.1016/S1473-3099(19)30738-8. Epub 2020 May 19. Lancet Infect Dis. 2020. PMID: 32442523 Clinical Trial.
-
Immunogenicity of sequential inactivated and oral poliovirus vaccines (OPV) versus inactivated poliovirus vaccine (IPV) alone in healthy infants: A systematic review and meta-analysis.Hum Vaccin Immunother. 2018;14(11):2636-2643. doi: 10.1080/21645515.2018.1489188. Epub 2018 Jul 16. Hum Vaccin Immunother. 2018. PMID: 29985751 Free PMC article.
-
Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule.Vaccine. 2017 May 19;35(22):2993-2998. doi: 10.1016/j.vaccine.2017.03.008. Epub 2017 Apr 20. Vaccine. 2017. PMID: 28434691 Free PMC article. Review.
Cited by
-
Serological investigation on the prevalence of poliovirus in Guangdong province: A cross-sectional study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2300156. doi: 10.1080/21645515.2023.2300156. Epub 2024 Jan 8. Hum Vaccin Immunother. 2024. PMID: 38189143 Free PMC article.
-
Relationship Between Helicobacter pylori IgG Seroprevalence and the Immune Response to Poliovirus Vaccine Among School-Age Children From a Population With Near-Universal Immunity Level.Front Med (Lausanne). 2022 Jan 20;8:797719. doi: 10.3389/fmed.2021.797719. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35127752 Free PMC article.
-
Quantitative Analysis of the Instant and Persistent Inhibition Effects of Maternal Poliovirus Antibodies on the Immune Response in a Phase IV Trial of a Sabin Strain-Based Inactivated Poliovirus Vaccine.Vaccines (Basel). 2024 Feb 19;12(2):217. doi: 10.3390/vaccines12020217. Vaccines (Basel). 2024. PMID: 38400200 Free PMC article.
-
Safety and immunogenicity of 3 formulations of a Sabin inactivated poliovirus vaccine produced on the PER.C6® cell line: A phase 2, double-blind, randomized, controlled study in infants vaccinated at 6, 10 and 14 weeks of age.Hum Vaccin Immunother. 2022 Nov 30;18(5):2044255. doi: 10.1080/21645515.2022.2044255. Epub 2022 Mar 28. Hum Vaccin Immunother. 2022. PMID: 35344464 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Polio eradication. https://www.who.int/news-room/facts-in-pictures/detail/polio-eradication. Accessed 10 Jan 2020.
-
- World Health Organization. Two out of three wild poliovirus strains eradicated. https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wi.... Accessed 15 Jan 2020.
-
- World Health Organization. Global Polio Eradication Initiative. http://polioeradication.org/polio-today/faq/. Accessed 2 Feb 2020.
-
- Centers for Disease Control and Prevention. What are the Types of Polio Vaccine? https://www.cdc.gov/vaccines/vpd/polio/public/. Accessed 10 Feb 2020.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

