Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice
- PMID: 32867778
- PMCID: PMC7457519
- DOI: 10.1186/s12940-020-00614-w
Perinatal oral exposure to low doses of bisphenol A, S or F impairs immune functions at intestinal and systemic levels in female offspring mice
Abstract
Background: Bisphenol A (BPA), one of the highest-volume chemicals produced worldwide, has been identified as an endocrine disruptor. Many peer-reviewing studies have reported adverse effects of low dose BPA exposure, particularly during perinatal period (gestation and/or lactation). We previously demonstrated that perinatal oral exposure to BPA (via gavage of mothers during gestation and lactation) has long-term consequences on immune response and intestinal barrier functions. Due to its adverse effects on several developmental and physiological processes, BPA was removed from consumer products and replaced by chemical substitutes such as BPS or BPF, that are structurally similar and not well studied compare to BPA. Here, we aimed to compare perinatal oral exposure to these bisphenols (BPs) at two doses (5 and 50 μg/kg of body weight (BW)/day (d)) on immune response at intestinal and systemic levels in female offspring mice at adulthood (Post Natal Day PND70).
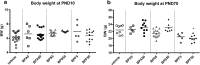
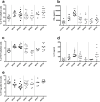
Methods: Pregnant female mice were orally exposed to BPA, BPS or BPF at 5 or 50 μg/kg BW/d from 15th day of gravidity to weaning of pups at Post-Natal Day (PND) 21. Humoral and cellular immune responses of adult offspring (PND70) were analysed at intestinal and systemic levels.
Results: In female offspring, perinatal oral BP exposure led to adverse effects on intestinal and systemic immune response that were dependant of the BP nature (A, S or F) and dose of exposure. Stronger impacts were observed with BPS at the dose of 5 μg/kg BW/d on inflammatory markers in feces associated with an increase of anti-E. coli IgG in plasma. BPA and BPF exposure induced prominent changes at low dose in offspring mice, in term of intestinal and systemic immune responses, provoking an intestinal and systemic Th1/Th17 inflammation.
Conclusion: These findings provide, for the first time, results of long-time consequences of BPA, S and F perinatal exposure by oral route on immune response in offspring mice. This work warns that it is mandatory to consider immune markers and dose exposure in risk assessment associated to new BPA's alternatives.
Keywords: Bisphenol A; Bisphenol F; Bisphenol S; Cytokines; Immune responses; Immunoglobulin; Intestine; Perinatal exposure; Th1/Th17.
Conflict of interest statement
Not applicable.
Figures




Similar articles
-
Bisphenol A, S or F mother's dermal impregnation impairs offspring immune responses in a dose and sex-specific manner in mice.Sci Rep. 2021 Jan 18;11(1):1650. doi: 10.1038/s41598-021-81231-6. Sci Rep. 2021. PMID: 33462300 Free PMC article.
-
Oral exposure to bisphenols induced food intolerance and colitis in vivo by modulating immune response in adult mice.Food Chem Toxicol. 2020 Dec;146:111773. doi: 10.1016/j.fct.2020.111773. Epub 2020 Oct 1. Food Chem Toxicol. 2020. PMID: 33011352
-
Perinatal exposure to a low dose of bisphenol A impaired systemic cellular immune response and predisposes young rats to intestinal parasitic infection.PLoS One. 2014 Nov 21;9(11):e112752. doi: 10.1371/journal.pone.0112752. eCollection 2014. PLoS One. 2014. PMID: 25415191 Free PMC article.
-
A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound.Fertil Steril. 2015 Jan;103(1):11-21. doi: 10.1016/j.fertnstert.2014.11.005. Epub 2014 Dec 2. Fertil Steril. 2015. PMID: 25475787 Review.
-
Impact of Bisphenol A and its alternatives on oocyte health: a scoping review.Hum Reprod Update. 2024 Dec 1;30(6):653-691. doi: 10.1093/humupd/dmae025. Hum Reprod Update. 2024. PMID: 39277428 Free PMC article.
Cited by
-
Short-Half-Life Chemicals: Maternal Exposure and Offspring Health Consequences-The Case of Synthetic Phenols, Parabens, and Phthalates.Toxics. 2024 Sep 29;12(10):710. doi: 10.3390/toxics12100710. Toxics. 2024. PMID: 39453131 Free PMC article. Review.
-
The Endocrine Disruptor Compound Bisphenol-A (BPA) Regulates the Intra-Tumoral Immune Microenvironment and Increases Lung Metastasis in an Experimental Model of Breast Cancer.Int J Mol Sci. 2022 Feb 25;23(5):2523. doi: 10.3390/ijms23052523. Int J Mol Sci. 2022. PMID: 35269666 Free PMC article.
-
Early Life Exposure to Food Contaminants and Social Stress as Risk Factor for Metabolic Disorders Occurrence?-An Overview.Biomolecules. 2021 May 3;11(5):687. doi: 10.3390/biom11050687. Biomolecules. 2021. PMID: 34063694 Free PMC article. Review.
-
Exposure to a mixture of 23 chemicals associated with unconventional oil and gas operations alters immune response to challenge in adult mice.J Immunotoxicol. 2021 Dec;18(1):105-117. doi: 10.1080/1547691X.2021.1965677. J Immunotoxicol. 2021. PMID: 34455897 Free PMC article.
-
Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells.Front Pharmacol. 2021 Sep 21;12:743991. doi: 10.3389/fphar.2021.743991. eCollection 2021. Front Pharmacol. 2021. PMID: 34621174 Free PMC article.
References
-
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Opinion on BPA. EFSA J. 2015;13:3978. doi: 10.2903/j.efsa.2015.3978. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

