AKT/FOXO1 axis links cross-talking of endothelial cell and pericyte in TIE2-mutated venous malformations
- PMID: 32867785
- PMCID: PMC7457504
- DOI: 10.1186/s12964-020-00606-w
AKT/FOXO1 axis links cross-talking of endothelial cell and pericyte in TIE2-mutated venous malformations
Abstract
Background: Venous malformations (VMs), most of which associated with activating mutations in the endothelial cells (ECs) tyrosine kinase receptor TIE2, are characterized by dilated and immature veins with scarce smooth muscle cells (SMCs) coverage. However, the underlying mechanism of interaction between ECs and SMCs responsible for VMs has not been fully understood.
Methods: Here, we screened 5 patients with TIE2-L914F mutation who were diagnosed with VMs by SNP sequencing, and we compared the expression of platelet-derived growth factor beta (PDGFB) and α-SMA in TIE2 mutant veins and normal veins by immunohistochemistry. In vitro, we generated TIE2-L914F-expressing human umbilical vein endothelial cells (HUVECs) and performed BrdU, CCK-8, transwell and tube formation experiments on none-transfected and transfected ECs. Then we investigated the effects of rapamycin (RAPA) on cellular characteristics. Next we established a co-culture system and investigated the role of AKT/FOXO1/PDGFB in regulating cross-talking of mutant ECs and SMCs.
Results: VMs with TIE2-L914F mutation showed lower expression of PDGFB and α-SMA than normal veins. TIE2 mutant ECs revealed enhanced cell viability and motility, and decreased tube formation, whereas these phenotypes could be reversed by rapamycin. Mechanically, RAPA ameliorated the physiological function of mutant ECs by inhibiting AKT-mTOR pathway, but also facilitated the nuclear location of FOXO1 and the expression of PDGFB in mutant ECs, and then improved paracrine interactions between ECs and SMCs. Moreover, TIE2 mutant ECs strongly accelerated the transition of SMCs from contractile phenotype to synthetic phenotype, whereas RAPA could prevent the phenotype transition of SMCs.
Conclusions: Our data demonstrate a previously unknown mechanistic linkage of AKT-mTOR/FOXO1 pathway between mutant ECs and SMCs in modulating venous dysmorphogenesis, and AKT/FOXO1 axis might be a potential therapeutic target for the recovery of TIE2-mutation causing VMs. Video Abstract.
Keywords: AKT; Endothelial cells; FOXO1; Smooth muscle cells; TIE2-L914F; Venous malformation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



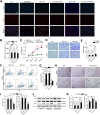
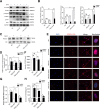

Similar articles
-
Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects.J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. J Clin Invest. 2015. PMID: 26258417 Free PMC article. Clinical Trial.
-
Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB.Hum Mol Genet. 2013 Sep 1;22(17):3438-48. doi: 10.1093/hmg/ddt198. Epub 2013 Apr 30. Hum Mol Genet. 2013. PMID: 23633549 Free PMC article.
-
Ponatinib Combined With Rapamycin Causes Regression of Murine Venous Malformation.Arterioscler Thromb Vasc Biol. 2019 Mar;39(3):496-512. doi: 10.1161/ATVBAHA.118.312315. Arterioscler Thromb Vasc Biol. 2019. PMID: 30626204 Free PMC article.
-
Review of the endothelial pathogenic mechanism of TIE2-related venous malformation.J Vasc Surg Venous Lymphat Disord. 2017 Sep;5(5):740-748. doi: 10.1016/j.jvsv.2017.05.001. Epub 2017 May 16. J Vasc Surg Venous Lymphat Disord. 2017. PMID: 28818232 Review.
-
Genetic landscape of common venous malformations in the head and neck.J Vasc Surg Venous Lymphat Disord. 2021 Jul;9(4):1007-1016.e7. doi: 10.1016/j.jvsv.2020.11.016. Epub 2020 Nov 26. J Vasc Surg Venous Lymphat Disord. 2021. PMID: 33248299 Review.
Cited by
-
Endothelial TIE2 Mutation Induced Contraction Deficiency of Vascular Smooth Muscle Cells via Phenotypic Transition Regulation in Venous Malformations.Int J Med Sci. 2025 May 7;22(10):2518-2532. doi: 10.7150/ijms.102700. eCollection 2025. Int J Med Sci. 2025. PMID: 40386049 Free PMC article.
-
A histological study of vascular wall resident stem cells in venous malformations.Cell Tissue Res. 2022 Nov;390(2):229-243. doi: 10.1007/s00441-022-03672-3. Epub 2022 Aug 2. Cell Tissue Res. 2022. PMID: 35916917
-
Macrophage miR-149-5p induction is a key driver and therapeutic target for BRONJ.JCI Insight. 2022 Aug 22;7(16):e159865. doi: 10.1172/jci.insight.159865. JCI Insight. 2022. PMID: 35993364 Free PMC article.
-
What the Interventional Radiologist Needs to Know about the Genetics of Vascular Anomalies.Semin Intervent Radiol. 2024 Nov 7;41(4):350-362. doi: 10.1055/s-0044-1791204. eCollection 2024 Aug. Semin Intervent Radiol. 2024. PMID: 39524236 Review.
-
The Genetic Architecture of Vascular Anomalies: Current Data and Future Therapeutic Perspectives Correlated with Molecular Mechanisms.Int J Mol Sci. 2022 Oct 13;23(20):12199. doi: 10.3390/ijms232012199. Int J Mol Sci. 2022. PMID: 36293054 Free PMC article. Review.
References
-
- De Maria L, De Sanctis P, Tollefson M, Mardini S, Garrity JA, Morris PP, et al. Sclerotherapy for low-flow vascular malformations of the orbital and periocular region: systematic review and meta-analysis. Surv Ophthalmol. 2019;65(1):41–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

