Effects of oral contraceptives on the quality of life of women with polycystic ovary syndrome: a crossover randomized controlled trial
- PMID: 32867790
- PMCID: PMC7460764
- DOI: 10.1186/s12955-020-01544-4
Effects of oral contraceptives on the quality of life of women with polycystic ovary syndrome: a crossover randomized controlled trial
Abstract
Background and objective: A limited number of studies have evaluated the effects of oral contraceptives (OCs) on the quality of life (QOL) of polycystic ovary syndrome (PCOS) patients. This study aimed to compare the effects of using OCs containing levonorgestrel (LNG) and those containing desogestrel (DSG), cyproterone acetate (CPA) or drospirenone (DRSP) for 6 months on the QOL with PCOS.
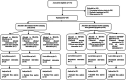
Methods: In this crossover randomized controlled 6-arm trial, 200 eligible patients with PCOS scheduled for OC therapy were randomly assigned to one of the 6 study arms. All 6 arms include two 6-month treatment periods, one period with OCs containing LNG, and the other with each of the 3 OCs containing DSG, CPA, or DRSP. Outcomes of interest were the total score of QOL and its domains, which were assessed using a specific and valid health-related quality of life questionnaire for PCOS, which is consisted of six domains, including psychosocial-emotional, self-image, fertility, sexual function, hirsutism, and obesity- menstrual disorders.
Results: Finally, a total of 88 patients were analyzed for this study. The results showed that use of OCs containing DSG, CPA, and DRSP for 3 months was not associated with significant differences in the total scores of QOL compared to those OCs containing LNG, whereas, after 6 months of treatment, patients treated with OCs containing CPA had more improvements in their total scores of QOL, in comparison to OCs containing LNG (P < 0.042). We found no significant differences in QoL domains, including psychosocial-emotional, self-image, fertility, sexual function, hirsutism, and obesity-menstrual disorders after 3-6 months of treatment with DSG, CPA, or DRSP, compared to LNG. The sequence and period effects were not significant in any of the analyses at 3 and 6 months of treatment. The carry-over effect was not significant for most outcomes assessed.
Conclusions: This crossover study demonstrated non-inferiority of OCs with newer generation progestins on different domains of QOL, in comparison with older compounds, although usage of products containing CPA was significantly associated with more improvement in total QOL of PCOS patients, compared to those containing LNG after 6-month of treatment.
Trial registration: IRCT201702071281N2 .
Keywords: Oral contraceptive (OC); Polycystic ovary syndrome (PCOS); Quality of life (QOL).
Conflict of interest statement
Authors have no conflict of interest to declare.
Figures
Similar articles
-
A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial.Hum Reprod. 2020 Jan 1;35(1):175-186. doi: 10.1093/humrep/dez255. Hum Reprod. 2020. PMID: 31916574 Clinical Trial.
-
Effects of oral contraceptives on serum concentrations of adipokines and adiposity indices of women with polycystic ovary syndrome: a randomized controlled trial.J Endocrinol Invest. 2021 Mar;44(3):567-580. doi: 10.1007/s40618-020-01349-8. Epub 2020 Jul 17. J Endocrinol Invest. 2021. PMID: 32681463 Clinical Trial.
-
Effects of oral contraceptives on metabolic profile in women with polycystic ovary syndrome: A meta-analysis comparing products containing cyproterone acetate with third generation progestins.Metabolism. 2017 Aug;73:22-35. doi: 10.1016/j.metabol.2017.05.001. Epub 2017 May 10. Metabolism. 2017. PMID: 28732568 Review.
-
Comparing the Effects of Oral Contraceptives Containing Levonorgestrel With Products Containing Antiandrogenic Progestins on Clinical, Hormonal, and Metabolic Parameters and Quality of Life in Women With Polycystic Ovary Syndrome: Crossover Randomized Controlled Trial Protocol.JMIR Res Protoc. 2017 Sep 29;6(9):e191. doi: 10.2196/resprot.8631. JMIR Res Protoc. 2017. PMID: 28963092 Free PMC article.
-
Effect of chlormadinone acetate versus drospirenone-containing oral contraceptives on the endocrinal features of women with polycystic ovary syndrome: Systematic review and meta-analysis of randomized clinical trials.J Gynecol Obstet Hum Reprod. 2019 Nov;48(9):763-770. doi: 10.1016/j.jogoh.2019.03.025. Epub 2019 Mar 30. J Gynecol Obstet Hum Reprod. 2019. PMID: 30940512
Cited by
-
Family planning and nutrition: systematic review of the effects of family planning on nutritional status of adolescent girls and women of reproductive age.BMJ Glob Health. 2025 Apr 24;10(Suppl 1):e015734. doi: 10.1136/bmjgh-2024-015734. BMJ Glob Health. 2025. PMID: 40280601 Free PMC article.
-
Combined oral contraceptive use and obesity in women with polycystic ovary syndrome. A meta-analysis of randomized clinical trials.Arch Gynecol Obstet. 2024 Oct;310(4):2223-2233. doi: 10.1007/s00404-024-07637-5. Epub 2024 Jul 18. Arch Gynecol Obstet. 2024. PMID: 39026022
-
Depressive Symptoms and Control of Emotions among Polish Women with Polycystic Ovary Syndrome.Int J Environ Res Public Health. 2022 Dec 15;19(24):16871. doi: 10.3390/ijerph192416871. Int J Environ Res Public Health. 2022. PMID: 36554751 Free PMC article.
-
Heavy menstrual bleeding due to primary myelofibrosis in a woman: a case report.Am J Transl Res. 2021 Oct 15;13(10):12016-12020. eCollection 2021. Am J Transl Res. 2021. PMID: 34786136 Free PMC article.
-
Mental Health Across the Menstrual Cycle in Polycystic Ovary Syndrome: Insights and Implications.Curr Psychiatry Rep. 2024 Nov;26(11):553-562. doi: 10.1007/s11920-024-01529-w. Epub 2024 Aug 31. Curr Psychiatry Rep. 2024. PMID: 39214948 Free PMC article. Review.
References
-
- Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson PA, Christman GM, Huang H, Hansen KR, Baker V. Sexual function in infertile women with polycystic ovary syndrome and unexplained infertility. Am J Obstet Gynecol. 2017;217:191. e191–191. e119. doi: 10.1016/j.ajog.2017.04.034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

