MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation
- PMID: 32867797
- PMCID: PMC7457538
- DOI: 10.1186/s12974-020-01933-y
MCC950, a selective NLPR3 inflammasome inhibitor, improves neurologic function and survival after cardiac arrest and resuscitation
Abstract
Background: Cardiac arrest (CA) is associated with high morbidity and mortality, even after spontaneous circulation is re-established. This dire situation is partly due to post-CA syndrome for which no specific and effective intervention is available. One key component of post-CA syndrome is sterile inflammation, which affects various organs including the brain. A major effector of sterile inflammation is activated NLRP3 inflammasome, which leads to increased release of interleukin (IL)-1β. However, how NLRP3 inflammasome impacts neuroinflammation and neurologic outcome after CA is largely undefined.
Methods: Mice were subjected to a potassium-based murine CA and cardiopulmonary resuscitation (CPR) model. MCC950 was used to suppress activation of NLRP3 inflammasome after CA/CPR. Levels of protein and mRNA were examined by Western blotting and quantitative PCR, respectively. Immunologic changes were assessed by measuring cytokine expression and immune cell compositions. CA outcomes, including neurologic deficits, bacterial load in the lung, and survival rate, were evaluated.
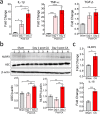
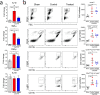
Results: Using our CA/CPR model, we found that NLRP3 inflammasome was activated in the post-CA brain, and that pro-inflammatory cytokine levels, including IL-1β, were increased. After treatment with MCC950, a potent and selective NLRP3 inflammasome inhibitor, mice exhibited improved functional recovery and survival rate during the 14-day observational period after CA/CPR. In line with these findings, IL-1β mRNA levels in the post-CA brain were significantly suppressed after MCC950 treatment. Interestingly, we also found that in MCC950- vs. vehicle-treated CA mice, immune homeostasis in the spleen was better preserved and bacterial load in the lung was significantly reduced.
Conclusions: Our data demonstrate that activation of NLRP3 inflammasome could be a key event shaping the post-CA immuno- and neuro-pathology, and identify this pathway as a unique and promising therapeutic target to improve outcomes after CA/CPR.
Keywords: CPR; Immunosuppression; Inflammasome; Ischemia/reperfusion; NLRP3; Neuroinflammation; Sterile inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Effects of NLRP3 inflammasome blockade on postresuscitation cerebral function in a rat model of cardiopulmonary resuscitation.Biomed Pharmacother. 2021 Nov;143:112093. doi: 10.1016/j.biopha.2021.112093. Epub 2021 Aug 30. Biomed Pharmacother. 2021. PMID: 34474352
-
Combined Therapy With Polyethylene Glycol-20k and MCC950 Preserves Post-Resuscitated Myocardial Function in a Rat Model of Cardiac Arrest and Cardiopulmonary Resuscitation.J Am Heart Assoc. 2021 May 4;10(9):e019177. doi: 10.1161/JAHA.120.019177. Epub 2021 Apr 22. J Am Heart Assoc. 2021. PMID: 33884887 Free PMC article.
-
Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury.Neurobiol Dis. 2018 Sep;117:15-27. doi: 10.1016/j.nbd.2018.05.016. Epub 2018 May 30. Neurobiol Dis. 2018. PMID: 29859317
-
MCC950 as a promising candidate for blocking NLRP3 inflammasome activation: A review of preclinical research and future directions.Arch Pharm (Weinheim). 2024 Nov;357(11):e2400459. doi: 10.1002/ardp.202400459. Epub 2024 Aug 23. Arch Pharm (Weinheim). 2024. PMID: 39180246 Review.
-
Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome.Eur J Pharmacol. 2022 Aug 5;928:175091. doi: 10.1016/j.ejphar.2022.175091. Epub 2022 Jun 14. Eur J Pharmacol. 2022. PMID: 35714692 Review.
Cited by
-
NLRP3 Inflammasome Inhibitors in Cardiovascular Diseases.Molecules. 2021 Feb 12;26(4):976. doi: 10.3390/molecules26040976. Molecules. 2021. PMID: 33673188 Free PMC article. Review.
-
Dying by fire: noncanonical functions of autophagy proteins in neuroinflammation and neurodegeneration.Neural Regen Res. 2022 Feb;17(2):246-250. doi: 10.4103/1673-5374.317958. Neural Regen Res. 2022. PMID: 34269183 Free PMC article. Review.
-
Flufenamic acid improves survival and neurologic outcome after successful cardiopulmonary resuscitation in mice.J Neuroinflammation. 2022 Sep 1;19(1):214. doi: 10.1186/s12974-022-02571-2. J Neuroinflammation. 2022. PMID: 36050694 Free PMC article.
-
Development and Evaluation of a Novel Mouse Model of Asphyxial Cardiac Arrest Revealed Severely Impaired Lymphopoiesis After Resuscitation.J Am Heart Assoc. 2021 Jun;10(11):e019142. doi: 10.1161/JAHA.120.019142. Epub 2021 May 20. J Am Heart Assoc. 2021. PMID: 34013738 Free PMC article.
-
Macrophage-NLRP3 Activation Promotes Right Ventricle Failure in Pulmonary Arterial Hypertension.Am J Respir Crit Care Med. 2022 Sep 1;206(5):608-624. doi: 10.1164/rccm.202110-2274OC. Am J Respir Crit Care Med. 2022. PMID: 35699679 Free PMC article.
References
-
- Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.CIR.0000023891.80661.AD. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

