KATANIN-dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in Arabidopsis
- PMID: 32867920
- PMCID: PMC7462616
- DOI: 10.7554/eLife.57282
KATANIN-dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in Arabidopsis
Abstract
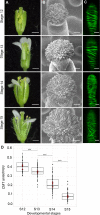
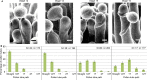
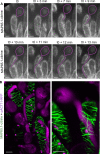
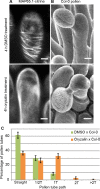
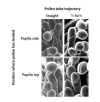
Successful fertilization in angiosperms depends on the proper trajectory of pollen tubes through the pistil tissues to reach the ovules. Pollen tubes first grow within the cell wall of the papilla cells, applying pressure to the cell. Mechanical forces are known to play a major role in plant cell shape by controlling the orientation of cortical microtubules (CMTs), which in turn mediate deposition of cellulose microfibrils (CMFs). Here, by combining imaging, genetic and chemical approaches, we show that isotropic reorientation of CMTs and CMFs in aged Col-0 and katanin1-5 (ktn1-5) papilla cells is accompanied by a tendency of pollen tubes to coil around the papillae. We show that this coiled phenotype is associated with specific mechanical properties of the cell walls that provide less resistance to pollen tube growth. Our results reveal an unexpected role for KTN1 in pollen tube guidance on the stigma by ensuring mechanical anisotropy of the papilla cell wall.
Keywords: A. thaliana; KATANIN; mechanical anisotropy; plant biology; pollen tube guidance; stigma papilla.
Plain language summary
Flowering plants produce small particles known as pollen that – with the help of the wind, bees and other animals – carry male sex cells (sperm) to female sex cells (eggs) contained within flowers. When a grain of pollen lands on the female organ of a flower, called the pistil, it gives rise to a tube that grows through the pistil towards the egg cells at the base. The surface of the pistil is covered in a layer of long cells named papillae. Like most plant cells, the papillae are surrounded by a rigid structure known as the cell wall, which is mainly composed of strands known as microfibrils. The pollen tube exerts pressure on a papilla to allow it to grow through the cell wall towards the base of the pistil. Previous studies have shown that the pistil produces signals that guide pollen tubes to the eggs. However, it remains unclear how pollen tubes orient themselves on the surface of papillae to grow in the right direction through the pistil. Riglet et al. combined microscopy, genetic and chemical approaches to study how pollen tubes grow through the surface of the pistils of a small weed known as Arabidopsis thaliana. The experiments showed that an enzyme called KATANIN conferred mechanical properties to the cell walls of papillae that allowed pollen tubes to grow towards the egg cells, and also altered the orientation of the microfibrils in these cell walls. In A. thaliana plants that were genetically modified to lack KATANIN the pollen tubes coiled around the papillae and sometimes grew in the opposite direction to where the eggs were. KATANIN is known to cut structural filaments inside the cells of plants, animals and most other living things. By revealing an additional role for KATANIN in regulating the mechanical properties of the papilla cell wall, these findings indicate this enzyme may also regulate the mechanical properties of cells involved in other biological processes.
© 2020, Riglet et al.
Conflict of interest statement
LR, FR, CK, SB, JS, IF, TG No competing interests declared
Figures

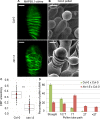

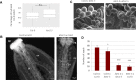
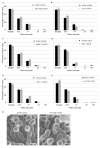
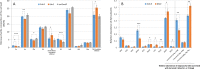


Comment in
-
Growing straight through walls.Elife. 2020 Sep 1;9:e61647. doi: 10.7554/eLife.61647. Elife. 2020. PMID: 32867921 Free PMC article.
Similar articles
-
Growing straight through walls.Elife. 2020 Sep 1;9:e61647. doi: 10.7554/eLife.61647. Elife. 2020. PMID: 32867921 Free PMC article.
-
KATANIN and cortical microtubule organization have a pivotal role in early pollen tube guidance.Plant Signal Behav. 2021 Aug 3;16(8):1921992. doi: 10.1080/15592324.2021.1921992. Epub 2021 May 7. Plant Signal Behav. 2021. PMID: 33960266 Free PMC article.
-
Geometric and mechanical guidance: Role of stigmatic epidermis in early pollen tube pathfinding in arabidopsis.PLoS Comput Biol. 2025 May 27;21(5):e1013077. doi: 10.1371/journal.pcbi.1013077. eCollection 2025 May. PLoS Comput Biol. 2025. PMID: 40424465 Free PMC article.
-
Functional genomics of pollen tube-pistil interactions in Arabidopsis.Biochem Soc Trans. 2010 Apr;38(2):593-7. doi: 10.1042/BST0380593. Biochem Soc Trans. 2010. PMID: 20298227 Review.
-
What are the key mechanisms that alter the morphology of stigmatic papillae in Arabidopsis thaliana?Plant Signal Behav. 2021 Dec 2;16(12):1980999. doi: 10.1080/15592324.2021.1980999. Epub 2021 Sep 22. Plant Signal Behav. 2021. PMID: 34549683 Free PMC article. Review.
Cited by
-
Signaling at Physical Barriers during Pollen-Pistil Interactions.Int J Mol Sci. 2021 Nov 12;22(22):12230. doi: 10.3390/ijms222212230. Int J Mol Sci. 2021. PMID: 34830110 Free PMC article. Review.
-
Maize stigmas react differently to self- and cross-pollination and fungal invasion.Plant Physiol. 2024 Dec 2;196(4):3071-3090. doi: 10.1093/plphys/kiae536. Plant Physiol. 2024. PMID: 39371027 Free PMC article.
-
Growing straight through walls.Elife. 2020 Sep 1;9:e61647. doi: 10.7554/eLife.61647. Elife. 2020. PMID: 32867921 Free PMC article.
-
A Complex Journey: Cell Wall Remodeling, Interactions, and Integrity During Pollen Tube Growth.Front Plant Sci. 2020 Nov 30;11:599247. doi: 10.3389/fpls.2020.599247. eCollection 2020. Front Plant Sci. 2020. PMID: 33329663 Free PMC article. Review.
-
Microtubule-associated protein SlMAP70 interacts with IQ67-domain protein SlIQD21a to regulate fruit shape in tomato.Plant Cell. 2023 Nov 30;35(12):4266-4283. doi: 10.1093/plcell/koad231. Plant Cell. 2023. PMID: 37668409 Free PMC article.
References
-
- Altartouri B, Bidhendi AJ, Tani T, Suzuki J, Conrad C, Chebli Y, Liu N, Karunakaran C, Scarcelli G, Geitmann A. Pectin chemistry and cellulose crystallinity govern pavement cell morphogenesis in a Multi-Step mechanism. Plant Physiology. 2019;181:127–141. doi: 10.1104/pp.19.00303. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

