A SARS-CoV-2 vaccine candidate would likely match all currently circulating variants
- PMID: 32868447
- PMCID: PMC7519301
- DOI: 10.1073/pnas.2008281117
A SARS-CoV-2 vaccine candidate would likely match all currently circulating variants
Abstract
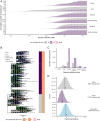
The magnitude of the COVID-19 pandemic underscores the urgency for a safe and effective vaccine. Many vaccine candidates focus on the Spike protein, as it is targeted by neutralizing antibodies and plays a key role in viral entry. Here we investigate the diversity seen in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequences and compare it to the sequence on which most vaccine candidates are based. Using 18,514 sequences, we perform phylogenetic, population genetics, and structural bioinformatics analyses. We find limited diversity across SARS-CoV-2 genomes: Only 11 sites show polymorphisms in >5% of sequences; yet two mutations, including the D614G mutation in Spike, have already become consensus. Because SARS-CoV-2 is being transmitted more rapidly than it evolves, the viral population is becoming more homogeneous, with a median of seven nucleotide substitutions between genomes. There is evidence of purifying selection but little evidence of diversifying selection, with substitution rates comparable across structural versus nonstructural genes. Finally, the Wuhan-Hu-1 reference sequence for the Spike protein, which is the basis for different vaccine candidates, matches optimized vaccine inserts, being identical to an ancestral sequence and one mutation away from the consensus. While the rapid spread of the D614G mutation warrants further study, our results indicate that drift and bottleneck events can explain the minimal diversity found among SARS-CoV-2 sequences. These findings suggest that a single vaccine candidate should be efficacious against currently circulating lineages.
Keywords: SARS-CoV-2; evolution; vaccine.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Low genetic diversity may be an Achilles heel of SARS-CoV-2.Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):24614-24616. doi: 10.1073/pnas.2017726117. Epub 2020 Sep 21. Proc Natl Acad Sci U S A. 2020. PMID: 32958678 Free PMC article. No abstract available.
References
-
- Wu F., et al. , Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv:10.1101/2020.03.30.20047365 (20 April 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

