Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches
- PMID: 32868492
- PMCID: PMC7530368
- DOI: 10.23876/j.krcp.20.082
Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches
Abstract
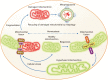
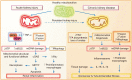
Mitochondria are energy-producing organelles that not only satisfy the high metabolic demands of the kidney but sense and respond to kidney injury-induced oxidative stress and inflammation. Kidneys are rich in mitochondria. Mitochondrial dysfunction plays a critical role in the progression of acute kidney injury and chronic kidney disease. Mitochondrial responses to specific stimuli are highly regulated and synergistically modulated by tightly interconnected processes, including mitochondrial dynamics (fission, fusion) and mitophagy. The counterbalance between these processes is essential in maintaining a healthy network of mitochondria. Recent literature suggests that alterations in mitochondrial dynamics are implicated in kidney injury and the progression of kidney diseases. A decrease in mitochondrial fusion promotes fission-induced mitochondrial fragmentation, but a reduction in mitochondrial fission produces excessive mitochondrial elongation. The removal of dysfunctional mitochondria by mitophagy is crucial for their quality control. Defective mitochondrial function disrupts cellular redox potential and can cause cell death. Mitochondrial DNA derived from damaged cells also act as damage-associated molecular patterns to recruit immune cells and the inflammatory response can further exaggerate kidney injury. This review provides a comprehensive overview of the role of mitochondrial dysfunction in acute kidney injury and chronic kidney disease. We discuss the processes that control mitochondrial stress responses to kidney injury and review recent advances in understanding the role of mitochondrial dysfunction in inflammation and tissue damage through the use of different experimental models of kidney disease. We also describe potential mitochondria-targeted therapeutic approaches.
Keywords: Acute kidney injury; Inflammation; Kidney diseases; Mitochondria; Oxidative stress.
Conflict of interest statement
The spouse of Mary E. Choi is a cofounder and shareholder and serves on the Scientific Advisory Board of Proterris, Inc. The remaining authors have no conflicts of interest to declare.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

