A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice
- PMID: 32868932
- PMCID: PMC7898783
- DOI: 10.1038/s41593-020-0695-6
A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice
Abstract
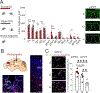
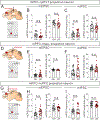
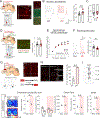
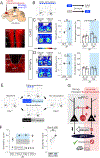
Juvenile social isolation reduces sociability in adulthood, but the underlying neural circuit mechanisms are poorly understood. We found that, in male mice, 2 weeks of social isolation immediately following weaning leads to a failure to activate medial prefrontal cortex neurons projecting to the posterior paraventricular thalamus (mPFC→pPVT) during social exposure in adulthood. Chemogenetic or optogenetic suppression of mPFC→pPVT activity in adulthood was sufficient to induce sociability deficits without affecting anxiety-related behaviors or preference toward rewarding food. Juvenile isolation led to both reduced excitability of mPFC→pPVT neurons and increased inhibitory input drive from low-threshold-spiking somatostatin interneurons in adulthood, suggesting a circuit mechanism underlying sociability deficits. Chemogenetic or optogenetic stimulation of mPFC→pPVT neurons in adulthood could rescue the sociability deficits caused by juvenile isolation. Our study identifies a pair of specific medial prefrontal cortex excitatory and inhibitory neuron populations required for sociability that are profoundly affected by juvenile social experience.
Conflict of interest statement
Figures


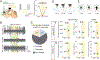

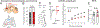








Comment in
-
Testing time alone.Nat Rev Neurosci. 2020 Nov;21(11):594. doi: 10.1038/s41583-020-00387-8. Nat Rev Neurosci. 2020. PMID: 32973313 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

