Novel β-Coronavirus (SARS-CoV-2): Current and future aspects of pharmacological treatments
- PMID: 32868970
- PMCID: PMC7449930
- DOI: 10.1016/j.jsps.2020.08.015
Novel β-Coronavirus (SARS-CoV-2): Current and future aspects of pharmacological treatments
Abstract
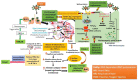
The novel coronavirus outbreak has reported to be rapidly spreading across the countries and becomes a foremost community health alarm. At present, no vaccine or specific drug is on hand for the treatment of this infectious disease. This review investigates the drugs, which are being evaluated and found to be effective against nCOVID-19 infection. A thorough literature search was performedon the recently published research papers in between January 2020 to May 2020, through various databases like "Science Direct", "Google Scholar", "PubMed","Medline", "Web of Science", and "World Health Organization (WHO)". We reviewed and documented the information related with the current and future aspects for the management and cure of COVID-19. As of 21st July 2020 a total of 14,562,550 confirmed cases of coronavirus and 607,781 deaths have been reported world-wide. The main clinical feature of COVID-19 ranges from asymptomatic disease to mild lower respiratory tract illness to severe pneumonia, acute lung injury, acute respiratory distress syndrome (ARDS), multiple organ dysfunction, and death. The drugs at present used in COVID-19 patients and ongoing clinical trials focusing on drug repurposing of various therapeutic classes of drug e.g. antiviral, anti-inflammatory and/or immunomodulatory drugs along with adjuvant/supportive care. Many drugs on clinical trials shows effective results on preliminary scale and now used currently in patients. Adjuvant/supportive care therapy are used in patients to get the best results in order to minimize the short and long-term complications. However, further studies and clinical trials are needed on large scale of population to reach any firm conclusion in terms of its efficacy and safety.
Keywords: Anti-inflammatory agents; Antiviral drugs; COVID-19; Corona virus; SARS-CoV-2.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Arnaud P. The interferons: pharmacology, mechanism of action, tolerance and side effects. Rev. Med. Interne. 2002;23:449s–458s. - PubMed
-
- ASH, American Society of COVID-19 and VTE/anticoagulation: frequently asked questions Version 2.1. Available at. Hematology. 2020
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous