Principles Learned from the International Race to Develop a Safe and Effective COVID-19 Vaccine
- PMID: 32868999
- PMCID: PMC7374935
- DOI: 10.1021/acscentsci.0c00644
Principles Learned from the International Race to Develop a Safe and Effective COVID-19 Vaccine
Abstract
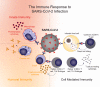
Vaccines against COVID-19 have the potential to protect people before they are exposed to the infective form of the virus. However, because of the involvement of pathogenic immune processes in many severe presentations of COVID-19, eliciting an immune response with a vaccine must strike a delicate balance to achieve viral clearance without also inducing immune-mediated harm. This Outlook synthesizes current laboratory findings to define which parts of the immune system help with recovery from and protection against the virus and which can lead to adverse outcomes. To inform our understanding, we analyze research about the immune mechanisms implicated in SARS-CoV, from the 2003 outbreak, and SARS-CoV-2, the virus causing COVID-19. The impact of how innate immunity, humoral immunity, and cell-mediated immunity play a role in a harmful versus helpful response is discussed, establishing principles to guide the development and evaluation of a safe but effective COVID-19 vaccine. The principles derived include (i) targeting the appropriate specificity and effector function of the humoral response, (ii) eliciting a T cell response, especially a cytotoxic T cell response, to achieve safe, yet effective, immune protection from COVID-19, and (iii) monitoring for the possibility of acute lung injury during SARS-CoV-2 infection post-vaccination in preclinical and clinical studies. These principles can not only guide efforts toward a safe and effective COVID-19 vaccine, but also the development of effective vaccines for viral pandemics to come.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- A Phase 2a, Randomized, Observer-Blind, Placebo Controlled, Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 SARS-COV-2 Vaccine in Adults Aged 18 Years and Older; Identifier, NCT04405076; ClinicalTrials.gov. U.S. National Library of Medicine, 2020.
-
- A 2-Part, Phase 1/2, Randomized, Observer-Blinded Study To Evaluate The Safety And Immunogenicity Of A SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine (SARS-CoV-2 rS) With Or Without MATRIX-M Adjuvant In Healthy Subjects; Identifier, NCT04368988; ClinicalTrials.gov. U.S. National Library of Medicine, 2020.
-
- A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-19 Using Different Dosing Regimens; Identifier, 2020-001038-36; EU Clinical Trials Register, 2020.
-
- A randomized, double-blinded, placebo-controlled phase II clinical trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector) in healthy adults aged above 18 years; Identifier, ChiCTR2000031781; Chinese Clinical Trial Register, 2020.
-
- Evaluation of the safety and immunogenicity of inactivated Novel Coronavirus Pneumonia (COVID-19) vaccine (Vero cells) in healthy population aged 6 years and above: a randomized, double-blind, placebo parallel-controlled phase I/II clinical trial; Identifier, ChiCTR2000031809; Chinese Clinical Trial Register, 2020.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

