This is a preprint.
Rethinking Remdesivir: Synthesis, Antiviral Activity and Pharmacokinetics of Oral Lipid Prodrugs
- PMID: 32869033
- PMCID: PMC7457622
- DOI: 10.1101/2020.08.26.269159
Rethinking Remdesivir: Synthesis, Antiviral Activity and Pharmacokinetics of Oral Lipid Prodrugs
Update in
-
Rethinking Remdesivir: Synthesis, Antiviral Activity, and Pharmacokinetics of Oral Lipid Prodrugs.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0115521. doi: 10.1128/AAC.01155-21. Epub 2021 Jul 26. Antimicrob Agents Chemother. 2021. PMID: 34310217 Free PMC article.
Abstract
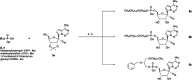
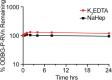
Remdesivir (RDV, GS-5734) is currently the only FDA-approved antiviral drug for the treatment of SARS CoV-2 infection. The drug is approved for use in adults or children 12-years or older who are hospitalized for the treatment of COVID-19 on the basis of an acceleration of clinical recovery for inpatients with this disease. Unfortunately, the drug must be administered intravenously, restricting its use to those requiring hospitalization for relatively advanced disease. RDV is also unstable in plasma and has a complex activation pathway which may contribute to its highly variable antiviral efficacy in SARS-CoV-2 infected cells. Potent orally bioavailable antiviral drugs for early treatment of SARS-CoV-2 infection are urgently needed and several including molnupiravir and PF-07321332 are currently in clinical development. We focused on making simple, orally bioavailable lipid analogs of Remdesivir nucleoside (RVn, GS-441524) that are processed to RVn-monophosphate, the precursor of the active RVn-triphosphate, by a single-step intracellular cleavage. In addition to high oral bioavailability, stability in plasma and simpler metabolic activation, new oral lipid prodrugs of RVn had submicromolar anti-SARS-CoV-2 activity in a variety of cell types including Vero E6, Calu-3, Caco-2, human pluripotent stem cell (PSC)-derived lung cells and Huh7.5 cells. In Syrian hamsters oral treatment with ODBG-P-RVn was well tolerated and achieved therapeutic levels in plasma above the EC90 for SARS-CoV-2. The results suggest further evaluation as an early oral treatment for SARS-CoV-2 infection to minimize severe disease and reduce hospitalizations.
Keywords: Caco-2 cells; Calu-3 cells; Huh7.5 cells and PSC-derived human lung cells; Remdesivir; Remdesivir nucleoside; SARS-CoV-2; Vero E6 cells; antiviral agents; lipid prodrugs.
Figures
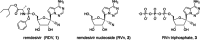




References
-
- Zhong NS, Zheng BJ, Li YM, Poon, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris, Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003. Oct 25;362(9393):1353–8. doi: 10.1016/s0140-6736(03)14630-2. - DOI - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020. Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. Epub 2020 Jan 24. - DOI - PMC - PubMed
-
- Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. Mar 16:ciaa270. doi: 10.1093/cid/ciaa270. Epub ahead of print. - DOI - PMC - PubMed
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. - DOI - PMC - PubMed
