Activation of astrocytes in hippocampus decreases fear memory through adenosine A1 receptors
- PMID: 32869747
- PMCID: PMC7505657
- DOI: 10.7554/eLife.57155
Activation of astrocytes in hippocampus decreases fear memory through adenosine A1 receptors
Abstract
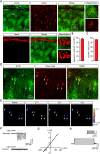
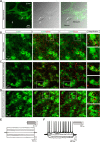
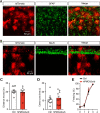
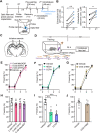
Astrocytes respond to and regulate neuronal activity, yet their role in mammalian behavior remains incompletely understood. Especially unclear is whether, and if so how, astrocyte activity regulates contextual fear memory, the dysregulation of which leads to pathological fear-related disorders. We generated GFAP-ChR2-EYFP rats to allow the specific activation of astrocytes in vivo by optogenetics. We found that after memory acquisition within a temporal window, astrocyte activation disrupted memory consolidation and persistently decreased contextual but not cued fear memory accompanied by reduced fear-related anxiety behavior. In vivo microdialysis experiments showed astrocyte photoactivation increased extracellular ATP and adenosine concentrations. Intracerebral blockade of adenosine A1 receptors (A1Rs) reversed the attenuation of fear memory. Furthermore, intracerebral or intraperitoneal injection of A1R agonist mimicked the effects of astrocyte activation. Therefore, our findings provide a deeper understanding of the astrocyte-mediated regulation of fear memory and suggest a new and important therapeutic strategy against pathological fear-related disorders.
Keywords: adenosine A1 receptors; anxiety; astrocyte; fear memory; memory consolidation; neuroscience; rat.
Plain language summary
Memory is the record of what we learn over time and is essential to our survival. But not all memories are helpful. Repeatedly recalling a traumatic event – such as an assault – can be harmful. About 1 in 3 people who experience severe trauma go on to develop post-traumatic stress disorder (PTSD), in which they re-live the traumatic event in the form of flashbacks and nightmares. Others develop panic disorder, phobias or depression. Preventing this chain of events is challenging because fear memories form rapidly and last a long time. Current treatments involve re-exposing individuals to the traumatic event. This could be real-life exposure in the case of a phobia. Or it could involve visualizing the event, in the case of PTSD. Controlled re-exposure can help individuals learn new coping strategies. But it does not erase the initial fear memory. A better approach might be to take advantage of the fact that new memories are unstable. To form a long-lasting memory trace, newly acquired information must go through a process called consolidation to stabilize it. This process takes place in an area of the brain called the hippocampus. If consolidation does not occur, new memory traces can fade away. Li, Li et al. now show that preventing consolidation in the rat brain stops the animals from forming lasting memories of a stressful event, namely a foot shock. In the study, the rats first learned to associate a foot shock with a tone. This training took place inside a specific chamber. After learning the association, the rats began to freeze – a sign of fear – whenever they entered the chamber. This happened even if the tone was not played. But Li, Li et al. showed that they could reduce this fear response by activating cells in the hippocampus known as astrocytes, shortly after the learning episode. Activating the astrocytes made them release a substance called adenosine. Molecules of adenosine then bound to and activated proteins called adenosine A1 receptors. Administering a drug that activated these receptors directly had the same effect as activating the astrocytes themselves. This suggests that drugs of this type could one day help patients with fear-related disorders such as PTSD and phobias. For this to become a reality, new studies must test different drugs and find the best ways of administering them. After testing in animal models, the next step will be preliminary clinical trials in people.
© 2020, Li et al.
Conflict of interest statement
YL, LL, JW, ZZ, XF, LQ, YZ, ZQ, SD, YY No competing interests declared
Figures








Similar articles
-
Noradrenergic projections from the locus coeruleus to the amygdala constrain fear memory reconsolidation.Elife. 2020 May 18;9:e57010. doi: 10.7554/eLife.57010. Elife. 2020. PMID: 32420872 Free PMC article.
-
The pivotal role of pituitary adenylate cyclase-activating polypeptide for lactate production and secretion in astrocytes during fear memory.Pharmacol Rep. 2021 Aug;73(4):1109-1121. doi: 10.1007/s43440-021-00222-6. Epub 2021 Apr 9. Pharmacol Rep. 2021. PMID: 33835466
-
Adenosine A1 receptor activation selectively impairs the acquisition of contextual fear conditioning in rats.Behav Neurosci. 2001 Dec;115(6):1283-90. doi: 10.1037//0735-7044.115.6.1283. Behav Neurosci. 2001. PMID: 11770059
-
[Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].Encephale. 2012 Oct;38(5):373-80. doi: 10.1016/j.encep.2011.12.003. Epub 2012 Jan 24. Encephale. 2012. PMID: 23062450 Review. French.
-
Consolidative mechanisms of emotional processing in REM sleep and PTSD.Sleep Med Rev. 2018 Oct;41:173-184. doi: 10.1016/j.smrv.2018.03.001. Epub 2018 Mar 15. Sleep Med Rev. 2018. PMID: 29628334 Review.
Cited by
-
Aberrant activation of hippocampal astrocytes causes neuroinflammation and cognitive decline in mice.PLoS Biol. 2024 Jul 11;22(7):e3002687. doi: 10.1371/journal.pbio.3002687. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38991663 Free PMC article.
-
Astrocytes in the Ventral Hippocampus Bidirectionally Regulate Innate and Stress-Induced Anxiety-Like Behaviors in Male Mice.Adv Sci (Weinh). 2024 Oct;11(38):e2400354. doi: 10.1002/advs.202400354. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39120568 Free PMC article.
-
Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala-Prefrontal Cortex Communication.Int J Mol Sci. 2022 May 29;23(11):6092. doi: 10.3390/ijms23116092. Int J Mol Sci. 2022. PMID: 35682767 Free PMC article.
-
PJA1 mediates the effects of astrocytic GPR30 on learning and memory in female mice.J Clin Invest. 2023 Sep 15;133(18):e165812. doi: 10.1172/JCI165812. J Clin Invest. 2023. PMID: 37712419 Free PMC article.
-
PTSD as an Endothelial Disease: Insights From COVID-19.Front Cell Neurosci. 2021 Oct 29;15:770387. doi: 10.3389/fncel.2021.770387. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34776871 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 2016YFC1306700/The National Key Research and Development Program/International
- 2016YFA0501000/The National Key Research and Development Program/International
- 2018PT31041/The Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences/International
- 2018B030331001/Science and technology Planning Project of Guangdong Province/International
- 2019FZA7009/Fundamental Research Funds for the Central Universitues/International
- 2016YFC1306700/National Key Research and Development Program/International
- 2016YFA0501000/National Key Research and Development Program/International
- 31970939/National Natural Science Foundation of China/International
- 81527901/National Natural Science Foundation of China/International
- 2018B030331001/Science and Technology Planning Project of Guangdong Province/International
- 31771167/National Natural Science Foundation of China/International
- 31571090/National Natural Science Foundation of China/International
- 81821091/National Natural Science Foundation of China/International
- 2019-I2M-5-057/CAMS Innovation Fund for Medical Science/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

