Lipid Profiles in Patients With Ulcerative Colitis Receiving Tofacitinib-Implications for Cardiovascular Risk and Patient Management
- PMID: 32870265
- PMCID: PMC8128390
- DOI: 10.1093/ibd/izaa227
Lipid Profiles in Patients With Ulcerative Colitis Receiving Tofacitinib-Implications for Cardiovascular Risk and Patient Management
Abstract
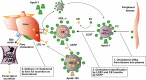
Background: Patients with ulcerative colitis (UC) are at elevated risk of cardiovascular disease vs the general population, despite a lower prevalence of traditional risk factors, including hyperlipidemia. Mechanistic studies in patients with rheumatoid arthritis and psoriasis suggest that tofacitinib restores serum lipids to preinflammation levels by reversing inflammation-induced cholesterol metabolism changes. We reviewed data on lipid levels and cardiovascular events, alongside recommendations for managing lipid levels during tofacitinib treatment in patients with UC, based on up-to-date expert guidelines.
Methods: Data were identified from a phase 3/open-label, long-term extension (OLE) tofacitinib UC clinical program (cutoff May 27, 2019). Literature was identified from PubMed (search terms "lipid," "cholesterol," "lipoprotein," "cardiovascular," "inflammation," "atherosclerosis," "tofacitinib," "rheumatoid arthritis," "psoriasis," "inflammatory bowel disease," "ulcerative colitis," "hyperlipidemia," and "guidelines") and author knowledge. Data were available from 4 phase 3 clinical trials of 1124 patients with moderately to severely active UC who received ≥1 dose of tofacitinib 5 or 10 mg twice daily in induction (two identical trials), maintenance, and OLE studies (treatment duration ≤6.8 years; 2576.4 patient-years of drug exposure).
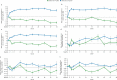
Results: In the OLE study, tofacitinib treatment was not associated with major changes from baseline in total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, total cholesterol/high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol/high-density lipoprotein cholesterol, with lipid levels and ratios generally remaining stable over time. The major adverse cardiovascular events incidence rate was 0.26/100 patient-years (95% confidence interval, 0.11-0.54).
Conclusions: Lipid levels and ratios remained generally unchanged from baseline in the OLE study after tofacitinib treatment, and major adverse cardiovascular events were infrequent. Long-term studies are ongoing.
Clinicaltrials.gov identifiers: NCT01465763, NCT01458951, NCT01458574, NCT01470612.
Keywords: clinical trials; lipids; tofacitinib.
© 2020 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures
Comment in
-
Lipid Profiles in Patients With Ulcerative Colitis Receiving Tofacitinib-Implications for Cardiovascular Risk and Patient Management.Inflamm Bowel Dis. 2021 Feb 16;27(3):e25. doi: 10.1093/ibd/izaa295. Inflamm Bowel Dis. 2021. PMID: 33155646 No abstract available.
Similar articles
-
Tofacitinib Treatment Is Associated With Modest and Reversible Increases in Serum Lipids in Patients With Ulcerative Colitis.Clin Gastroenterol Hepatol. 2020 Jan;18(1):123-132.e3. doi: 10.1016/j.cgh.2019.04.059. Epub 2019 May 8. Clin Gastroenterol Hepatol. 2020. PMID: 31077827
-
Tofacitinib, an Oral Janus Kinase Inhibitor: Analysis of Malignancy (Excluding Nonmelanoma Skin Cancer) Events Across the Ulcerative Colitis Clinical Program.Inflamm Bowel Dis. 2021 May 17;27(6):816-825. doi: 10.1093/ibd/izaa199. Inflamm Bowel Dis. 2021. PMID: 32766762 Free PMC article.
-
Changes in Lipid Levels and Incidence of Cardiovascular Events Following Tofacitinib Treatment in Patients With Psoriatic Arthritis: A Pooled Analysis Across Phase III and Long-Term Extension Studies.Arthritis Care Res (Hoboken). 2019 Oct;71(10):1387-1395. doi: 10.1002/acr.23930. Arthritis Care Res (Hoboken). 2019. PMID: 31112005 Free PMC article.
-
Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist.Semin Arthritis Rheum. 2016 Aug;46(1):71-80. doi: 10.1016/j.semarthrit.2016.03.004. Epub 2016 Mar 9. Semin Arthritis Rheum. 2016. PMID: 27079757 Review.
-
Efficacy and safety of biologic agents and tofacitinib in moderate-to-severe ulcerative colitis: A systematic overview of meta-analyses.United European Gastroenterol J. 2019 Dec;7(10):1285-1303. doi: 10.1177/2050640619883566. Epub 2019 Oct 17. United European Gastroenterol J. 2019. PMID: 31839954 Free PMC article.
Cited by
-
Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going.Drugs Context. 2022 Apr 8;11:2021-11-4. doi: 10.7573/dic.2021-11-4. eCollection 2022. Drugs Context. 2022. PMID: 35462642 Free PMC article. Review.
-
The Role of Adipocytes Recruited as Part of Tumor Microenvironment in Promoting Colorectal Cancer Metastases.Int J Mol Sci. 2024 Jul 30;25(15):8352. doi: 10.3390/ijms25158352. Int J Mol Sci. 2024. PMID: 39125923 Free PMC article. Review.
-
JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases.Front Med (Lausanne). 2023 Mar 2;10:1089099. doi: 10.3389/fmed.2023.1089099. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36936239 Free PMC article. Review.
-
Review article: guide to tofacitinib dosing in patients with ulcerative colitis.Aliment Pharmacol Ther. 2022 Oct;56(7):1131-1145. doi: 10.1111/apt.17185. Epub 2022 Aug 22. Aliment Pharmacol Ther. 2022. PMID: 35993338 Free PMC article. Review.
-
Risk of dyslipidemia and major adverse cardiac events with tofacitinib versus adalimumab in rheumatoid arthritis: a real-world cohort study from 7580 patients.Front Pharmacol. 2024 May 31;15:1370661. doi: 10.3389/fphar.2024.1370661. eCollection 2024. Front Pharmacol. 2024. PMID: 38881871 Free PMC article.
References
-
- Baena-Díez JM, Garcia-Gil M, Comas-Cufí M, et al. . Association between chronic immune-mediated inflammatory diseases and cardiovascular risk. Heart. 2018;104:119–126. - PubMed
-
- Kirchgesner J, Beaugerie L, Carrat F, et al. ; BERENICE study group . Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. 2018;67:1261–1268. - PubMed
-
- Rungoe C, Nyboe Andersen N, Jess T. Inflammatory bowel disease and risk of coronary heart disease. Trends Cardiovasc Med. 2015;25:699–704. - PubMed
-
- Singh S, Singh H, Loftus EV Jr, et al. . Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382–3 93.e1. - PubMed
-
- U.S. Food and Drug Administration. XELJANZ (tofacitinib): highlights for prescribing information. 2019. http://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed August 11, 2020.

