HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer
- PMID: 32870818
- PMCID: PMC7524491
- DOI: 10.1172/JCI137552
HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer
Abstract
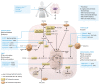
Hypoxia-inducible factors (HIFs) and the HIF-dependent cancer hallmarks angiogenesis and metabolic rewiring are well-established drivers of breast cancer aggressiveness, therapy resistance, and poor prognosis. Targeting of HIF and its downstream targets in angiogenesis and metabolism has been unsuccessful so far in the breast cancer clinical setting, with major unresolved challenges residing in target selection, development of robust biomarkers for response prediction, and understanding and harnessing of escape mechanisms. This Review discusses the pathophysiological role of HIFs, angiogenesis, and metabolism in breast cancer and the challenges of targeting these features in patients with breast cancer. Rational therapeutic combinations, especially with immunotherapy and endocrine therapy, seem most promising in the clinical exploitation of the intricate interplay of HIFs, angiogenesis, and metabolism in breast cancer cells and the tumor microenvironment.
Conflict of interest statement
Figures


References
-
- Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51(12):3316–3322. - PubMed
-
- Harris AL. Clinical strategies to inhibit tumor vascularization. In: Ribatti D, Pezzella F, eds. Tumor Vascularization. Elsevier Science; 2020:147–175.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

