LILRB3 (ILT5) is a myeloid cell checkpoint that elicits profound immunomodulation
- PMID: 32870822
- PMCID: PMC7526549
- DOI: 10.1172/jci.insight.141593
LILRB3 (ILT5) is a myeloid cell checkpoint that elicits profound immunomodulation
Abstract
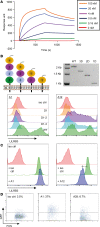
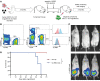
Despite advances in identifying the key immunoregulatory roles of many of the human leukocyte immunoglobulin-like receptor (LILR) family members, the function of the inhibitory molecule LILRB3 (ILT5, CD85a, LIR3) remains unclear. Studies indicate a predominant myeloid expression; however, high homology within the LILR family and a relative paucity of reagents have hindered progress toward identifying the function of this receptor. To investigate its function and potential immunomodulatory capacity, a panel of LILRB3-specific monoclonal antibodies (mAbs) was generated. LILRB3-specific mAbs bound to discrete epitopes in Ig-like domain 2 or 4. LILRB3 ligation on primary human monocytes by an agonistic mAb resulted in phenotypic and functional changes, leading to potent inhibition of immune responses in vitro, including significant reduction in T cell proliferation. Importantly, agonizing LILRB3 in humanized mice induced tolerance and permitted efficient engraftment of allogeneic cells. Our findings reveal powerful immunosuppressive functions of LILRB3 and identify it as an important myeloid checkpoint receptor.
Keywords: Immunology; Immunotherapy; Macrophages; Monocytes.
Conflict of interest statement
Figures



Similar articles
-
The Orphan Immune Receptor LILRB3 Modulates Fc Receptor-Mediated Functions of Neutrophils.J Immunol. 2020 Feb 15;204(4):954-966. doi: 10.4049/jimmunol.1900852. Epub 2020 Jan 8. J Immunol. 2020. PMID: 31915259 Free PMC article.
-
Copy number and nucleotide variation of the LILR family of myelomonocytic cell activating and inhibitory receptors.Immunogenetics. 2014 Feb;66(2):73-83. doi: 10.1007/s00251-013-0742-5. Epub 2013 Nov 21. Immunogenetics. 2014. PMID: 24257760 Free PMC article.
-
LILRB3 Supports Immunosuppressive Activity of Myeloid Cells and Tumor Development.Cancer Immunol Res. 2024 Mar 4;12(3):350-362. doi: 10.1158/2326-6066.CIR-23-0496. Cancer Immunol Res. 2024. PMID: 38113030 Free PMC article.
-
T-cell immunoglobulin and ITIM domain, as a potential immune checkpoint target for immunotherapy of colorectal cancer.IUBMB Life. 2021 May;73(5):726-738. doi: 10.1002/iub.2461. Epub 2021 Mar 30. IUBMB Life. 2021. PMID: 33686787 Review.
-
Chicken immunoregulatory Ig-like receptor families: an overview and expression details on ggTREM-A1.Dev Comp Immunol. 2013 Nov;41(3):403-12. doi: 10.1016/j.dci.2013.04.017. Epub 2013 May 3. Dev Comp Immunol. 2013. PMID: 23648646 Review.
Cited by
-
Distinct frequency patterns of LILRB3 and LILRA6 allelic variants in Europeans.Immunogenetics. 2023 Jun;75(3):263-267. doi: 10.1007/s00251-022-01286-1. Epub 2022 Nov 30. Immunogenetics. 2023. PMID: 36449053 Free PMC article.
-
Identification of novel immune-related biomarker and therapeutic drugs in Parkinson disease via integrated bioinformatics analysis.Medicine (Baltimore). 2023 Aug 4;102(31):e34456. doi: 10.1097/MD.0000000000034456. Medicine (Baltimore). 2023. PMID: 37543820 Free PMC article.
-
Hofbauer Cells Spread Listeria monocytogenes among Placental Cells and Undergo Pro-Inflammatory Reprogramming while Retaining Production of Tolerogenic Factors.mBio. 2021 Aug 31;12(4):e0184921. doi: 10.1128/mBio.01849-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399615 Free PMC article.
-
Multi-cohort cerebrospinal fluid proteomics identifies robust molecular signatures across the Alzheimer disease continuum.Neuron. 2025 May 7;113(9):1363-1379.e9. doi: 10.1016/j.neuron.2025.02.014. Epub 2025 Mar 14. Neuron. 2025. PMID: 40088886
-
Bipotential B-neutrophil progenitors are present in human and mouse bone marrow and emerge in the periphery upon stress hematopoiesis.mBio. 2024 Aug 14;15(8):e0159924. doi: 10.1128/mbio.01599-24. Epub 2024 Jul 16. mBio. 2024. PMID: 39012145 Free PMC article.
References
-
- Borges L, Hsu ML, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J Immunol. 1997;159(11):5192–5196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

