Gabapentin in pregnancy and the risk of adverse neonatal and maternal outcomes: A population-based cohort study nested in the US Medicaid Analytic eXtract dataset
- PMID: 32870921
- PMCID: PMC7462308
- DOI: 10.1371/journal.pmed.1003322
Gabapentin in pregnancy and the risk of adverse neonatal and maternal outcomes: A population-based cohort study nested in the US Medicaid Analytic eXtract dataset
Abstract
Background: Despite the widespread use, only sparse information is available on the safety of gabapentin during pregnancy. We sought to evaluate the association between gabapentin exposure during pregnancy and risk of adverse neonatal and maternal outcomes.
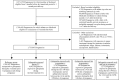
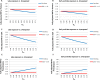
Methods and findings: Using the United States Medicaid Analytic eXtract (MAX) dataset, we conducted a population-based study of 1,753,865 Medicaid-eligible pregnancies between January 2000 and December 2013. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early and late in pregnancy. Gabapentin-unexposed pregnancies served as the reference. We estimated relative risks (RRs) and 95% confidence intervals (CIs) using fine stratification on the propensity score (PS) to control for over 70 confounders (e.g., maternal age, race/ethnicity, indications for gabapentin, other pain conditions, hypertension, diabetes, use of opioids, and specific morphine equivalents). We identified 4,642 pregnancies exposed in T1 (mean age = 28 years; 69% white), 3,745 exposed in early pregnancy only (28 years; 67% white), 556 exposed in late pregnancy only (27 years; 60% white), and 1,275 exposed in both early and late pregnancy (29 years; 75% white). The reference group consisted of 1,744,447 unexposed pregnancies (24 years; 40% white). The adjusted RR for major malformations was 1.07 (95% CI 0.94-1.21, p = 0.33) and for cardiac defects 1.12 (0.89-1.40, p = 0.35). Requiring ≥2 gabapentin dispensings moved the RR to 1.40 (1.03-1.90, p = 0.03) for cardiac defects. There was a higher risk of preterm birth among women exposed to gabapentin either late (RR, 1.28 [1.08-1.52], p < 0.01) or both early and late in pregnancy (RR, 1.22 [1.09-1.36], p < 0.001), SGA among women exposed to gabapentin early (1.17 [1.02-1.33], p = 0.02), late (1.39 [1.01-1.91], p = 0.05), or both early and late in pregnancy (RR, 1.32 [1.08-1.60], p < 0.01), and NICU admission among women exposed to gabapentin both early and late in pregnancy (RR, 1.35 [1.20-1.52], p < 0.001). There was no higher risk of preeclampsia among women exposed to gabapentin after adjustment. Study limitations include the potential for residual confounding and exposure misclassification.
Conclusions: In this large population-based study, we did not find evidence for an association between gabapentin exposure during early pregnancy and major malformations overall, although there was some evidence of a higher risk of cardiac malformations. Maternal use of gabapentin, particularly late in pregnancy, was associated with a higher risk of PTB, SGA, and NICUa.
Conflict of interest statement
We have read the journal's policy and the authors of this manuscript have the following competing interests: EP is investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to the topic of the submitted work. SH-D has consulted for Boehringer-Ingelheim and UCB for unrelated topics and has worked with the AED pregnancy registry, which is funded by multiple companies. KFH, BTB, and SH-D have been investigators on grants to the Brigham and Women’s Hospital from Lilly, GSK, and Pfizer and BTB on grants from Baxalta and Pacira, unrelated to the topic of this manuscript. BTB consults for Aetion for unrelated projects and was a consultant on a postpartum hemorrhage quality improvement project sponsored by a grant from Merck for Mothers. RJD reports grants from Merck, outside the submitted work.
Figures
References
-
- Gabapentin Clinical Pharmacology. Tampa (FL): Elsevier; 2012.
-
- Neurontin (gabapentin) package insert. New York: Pfizer, NY; 2017.
-
- Online L. Gabapentin: Drug Information. Hudson, Ohio: Lexi-Comp, Inc.
-
- Montouris G. Gabapentin exposure in human pregnancy: results from the Gabapentin Pregnancy Registry. Epilepsy & behavior: E&B. 2003;4:310–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

