Loss of CBX2 induces genome instability and senescence-associated chromosomal rearrangements
- PMID: 32870972
- PMCID: PMC7594495
- DOI: 10.1083/jcb.201910149
Loss of CBX2 induces genome instability and senescence-associated chromosomal rearrangements
Abstract
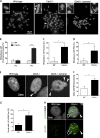
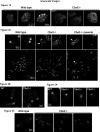
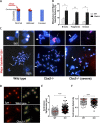
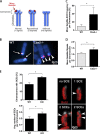
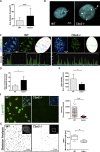
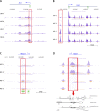
The polycomb group protein CBX2 is an important epigenetic reader involved in cell proliferation and differentiation. While CBX2 overexpression occurs in a wide range of human tumors, targeted deletion results in homeotic transformation, proliferative defects, and premature senescence. However, its cellular function(s) and whether it plays a role in maintenance of genome stability remain to be determined. Here, we demonstrate that loss of CBX2 in mouse fibroblasts induces abnormal large-scale chromatin structure and chromosome instability. Integrative transcriptome analysis and ATAC-seq revealed a significant dysregulation of transcripts involved in DNA repair, chromocenter formation, and tumorigenesis in addition to changes in chromatin accessibility of genes involved in lateral sclerosis, basal transcription factors, and folate metabolism. Notably, Cbx2-/- cells exhibit prominent decondensation of satellite DNA sequences at metaphase and increased sister chromatid recombination events leading to rampant chromosome instability. The presence of extensive centromere and telomere defects suggests a prominent role for CBX2 in heterochromatin homeostasis and the regulation of nuclear architecture.
© 2020 Baumann et al.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

