Live-attenuated Vaccines Prevent Respiratory Syncytial Virus-associated Illness in Young Children
- PMID: 32871092
- PMCID: PMC7924568
- DOI: 10.1164/rccm.202005-1660OC
Live-attenuated Vaccines Prevent Respiratory Syncytial Virus-associated Illness in Young Children
Abstract
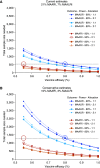
Rationale: Active immunization is needed to protect infants and young children against respiratory syncytial virus (RSV). Rationally designed live-attenuated RSV vaccines are in clinical development.Objectives: Develop preliminary estimates of vaccine efficacy, assess durability of antibody responses to vaccination and "booster" responses after natural RSV infection, and determine sample sizes needed for more precise estimates of vaccine efficacy.Methods: We analyzed data from seven phase 1 trials of live-attenuated RSV vaccines in 6- to 24-month-old children (n = 239).Measurements and Main Results: The five vaccine regimens that induced neutralizing antibody responses in ≥80% of vaccinees (defined post hoc as "more promising") protected against RSV-associated medically attended acute respiratory illness (RSV-MAARI) and medically attended acute lower respiratory illness (RSV-MAALRI) and primed for potent anamnestic responses upon natural exposure to wild-type RSV. Among recipients of "more promising" RSV vaccines, efficacy against RSV-MAARI was 67% (95% confidence interval [CI], 24 to 85; P = 0.008) and against RSV-MAALRI was 88% (95% CI, -9 to 99; P = 0.04). A greater than or equal to fourfold increase in RSV serum neutralizing antibody following vaccination was strongly associated with protection against RSV-MAARI (odds ratio, 0.26; 95% CI, 0.09 to 0.75; P = 0.014) and RSV-MAALRI; no child with a greater than or equal to fourfold increase developed RSV-MAALRI. Rates of RSV-MAARI and RSV-MAALRI in placebo recipients were 21% and 7%, respectively. Given these rates, a study of 540 RSV-naive children would have 90% power to demonstrate ≥55% efficacy against RSV-MAARI and ≥80% efficacy against RSV-MAALRI; if rates were 10% and 3%, a study of 1,300 RSV-naive children would be needed.Conclusions: Rapid development of a live-attenuated RSV vaccine could contribute substantially to reducing the global burden of RSV disease.
Keywords: RSV; efficacy; immunity; pediatric; vaccine.
Figures





Comment in
-
Live-attenuated Respiratory Syncytial Virus Vaccines: Time for the Next Step.Am J Respir Crit Care Med. 2021 Mar 1;203(5):538-539. doi: 10.1164/rccm.202009-3431ED. Am J Respir Crit Care Med. 2021. PMID: 32986467 Free PMC article. No abstract available.
References
-
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. - PMC - PubMed
-
- Karron RA, Zar HJ. Determining the outcomes of interventions to prevent respiratory syncytial virus disease in children: what to measure? Lancet Respir Med. 2018;6:65–74. - PubMed
-
- Mazur NI, Higgins D, Nunes MC, Melero JA, Langedijk AC, Horsley N, et al. Respiratory Syncytial Virus Network (ReSViNET) Foundation. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis. 2018;18:e295–e311. - PubMed
-
- Karron RA, Black RE. Determining the burden of respiratory syncytial virus disease: the known and the unknown. Lancet. 2017;390:917–918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

