Recent trends in protein and peptide-based biomaterials for advanced drug delivery
- PMID: 32871201
- PMCID: PMC7456198
- DOI: 10.1016/j.addr.2020.08.008
Recent trends in protein and peptide-based biomaterials for advanced drug delivery
Abstract
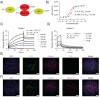
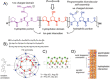
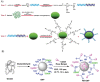
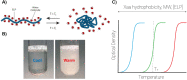
Engineering protein and peptide-based materials for drug delivery applications has gained momentum due to their biochemical and biophysical properties over synthetic materials, including biocompatibility, ease of synthesis and purification, tunability, scalability, and lack of toxicity. These biomolecules have been used to develop a host of drug delivery platforms, such as peptide- and protein-drug conjugates, injectable particles, and drug depots to deliver small molecule drugs, therapeutic proteins, and nucleic acids. In this review, we discuss progress in engineering the architecture and biological functions of peptide-based biomaterials -naturally derived, chemically synthesized and recombinant- with a focus on the molecular features that modulate their structure-function relationships for drug delivery.
Keywords: Bioinspired materials; Drug delivery; Hierarchical self-assembly; Polypeptides; Recombinant proteins.
Copyright © 2020. Published by Elsevier B.V.
Figures











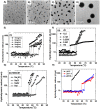
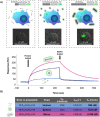
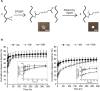

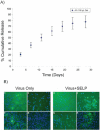

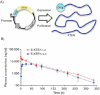
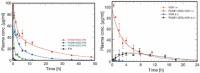
References
-
- Tong X., Pan W., Su T., Zhang M., Dong W., Qi X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020;148:104501. doi: 10.1016/j.reactfunctpolym.2020.104501. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

