Urea-Assisted Synthesis and Characterization of Saponite with Different Octahedral (Mg, Zn, Ni, Co) and Tetrahedral Metals (Al, Ga, B), a Review
- PMID: 32872173
- PMCID: PMC7555627
- DOI: 10.3390/life10090168
Urea-Assisted Synthesis and Characterization of Saponite with Different Octahedral (Mg, Zn, Ni, Co) and Tetrahedral Metals (Al, Ga, B), a Review
Abstract
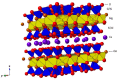
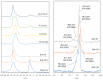
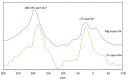
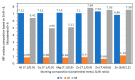
Clay minerals surfaces potentially play a role in prebiotic synthesis through adsorption of organic monomers that give rise to highly concentrated systems; facilitate condensation and polymerization reactions, protection of early biomolecules from hydrolysis and photolysis, and surface-templating for specific adsorption and synthesis of organic molecules. This review presents processes of clay formation using saponite as a model clay mineral, since it has been shown to catalyze organic reactions, is easy to synthesize in large and pure form, and has tunable properties. In particular, a method involving urea is presented as a reasonable analog of natural processes. The method involves a two-step process: (1) formation of the precursor aluminosilicate gel and (2) hydrolysis of a divalent metal (Mg, Ni, Co, and Zn) by the slow release of ammonia from urea decomposition. The aluminosilicate gels in the first step forms a 4-fold-coordinated Al3+ similar to what is found in nature such as in volcanic glass. The use of urea, a compound figuring in many prebiotic model reactions, circumvents the formation of undesirable brucite, Mg(OH)2, in the final product, by slowly releasing ammonia thereby controlling the hydrolysis of magnesium. In addition, the substitution of B and Ga for Si and Al in saponite is also described. The saponite products from this urea-assisted synthesis were tested as catalysts for several organic reactions, including Friedel-Crafts alkylation, cracking, and isomerization reactions.
Keywords: clay minerals; heterogeneous catalysis; saponite; synthesis; urea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bernal J.D. The Physical Basis of Life. Proc. Phys. Soc. Sect. B. 1949;62:597–618. doi: 10.1088/0370-1301/62/10/301. - DOI
-
- Cairns-Smith A.G., Hartman H. Clay Minerals and the Origin of Life. Cambridge University Press; Cambridge, UK: 1986.
-
- Balogh M., Laszlo P. Organic Chemistry Using Clays. Springer; Berlin/Heidelberg, Germany: 1993.
-
- Aldersley M.F., Joshi P.C., Price J.D., Ferris J.P. The role of montmorillonite in its catalysis of RNA synthesis. Appl. Clay Sci. 2011;54:1–14. doi: 10.1016/j.clay.2011.06.011. - DOI
Publication types
LinkOut - more resources
Full Text Sources

