Anti-Glucosylsphingosine Autoimmunity, JAK2V617F-Dependent Interleukin-1β and JAK2V617F-Independent Cytokines in Myeloproliferative Neoplasms
- PMID: 32872203
- PMCID: PMC7564615
- DOI: 10.3390/cancers12092446
Anti-Glucosylsphingosine Autoimmunity, JAK2V617F-Dependent Interleukin-1β and JAK2V617F-Independent Cytokines in Myeloproliferative Neoplasms
Abstract
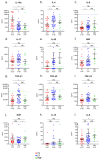
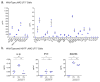
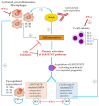
Inflammatory cytokines play a major role in myeloproliferative neoplasms (MPNs) as regulators of the MPN clone and as mediators of clinical symptoms and complications. Firstly, we investigated the effect of JAK2V617F on 42 molecules linked to inflammation. For JAK2V617F-mutated patients, the JAK2V617F allele burden (%JAK2V617F) correlated with the levels of IL-1β, IL-1Rα, IP-10 and leptin in polycythemia vera (PV), and with IL-33 in ET; for all other molecules, no correlation was found. Cytokine production was also studied in the human megakaryocytic cell line UT-7. Wild-type UT-7 cells secreted 27/42 cytokines measured. UT-7 clones expressing 50% or 75% JAK2V617F were generated, in which the production of IL-1β, IP-10 and RANTES was increased; other cytokines were not affected. Secondly, we searched for causes of chronic inflammation in MPNs other than driver mutations. Since antigen-driven selection is increasingly implicated in the pathogenesis of blood malignancies, we investigated whether proinflammatory glucosylsphingosine (GlcSph) may play a role in MPNs. We report that 20% (15/75) of MPN patients presented with anti-GlcSph IgGs, distinguished by elevated levels of 11 cytokines. In summary, only IL-1β and IP-10 were linked to JAK2V617F both in patients and in UT-7 cells; other inflammation-linked cytokines in excess in MPNs were not. For subsets of MPN patients, a possible cause of inflammation may be auto-immunity against glucolipids.
Keywords: CALR exon 9 mutants; CRISPR technology; IL-1Rα; IL-33; IP-10; JAK2V617F; UT-7; antigenic stimulation; auto-immunity; cytokines; glucolipids; glucosylsphingosine (GlcSph); inflammation; interleukin-1β (IL-1β); leptin; myeloproliferative neoplasms (MPNs).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Anti-inflammatory cytokines hepatocyte growth factor and interleukin-11 are over-expressed in Polycythemia vera and contribute to the growth of clonal erythroblasts independently of JAK2V617F.Oncogene. 2011 Feb 24;30(8):990-1001. doi: 10.1038/onc.2010.479. Epub 2010 Nov 1. Oncogene. 2011. PMID: 21042281
-
Proinflammatory Cytokine IL-6 and JAK-STAT Signaling Pathway in Myeloproliferative Neoplasms.Mediators Inflamm. 2015;2015:453020. doi: 10.1155/2015/453020. Epub 2015 Sep 29. Mediators Inflamm. 2015. PMID: 26491227 Free PMC article.
-
TLR4 and RAGE conversely mediate pro-inflammatory S100A8/9-mediated inhibition of proliferation-linked signaling in myeloproliferative neoplasms.Cell Oncol (Dordr). 2018 Oct;41(5):541-553. doi: 10.1007/s13402-018-0392-6. Epub 2018 Jun 26. Cell Oncol (Dordr). 2018. PMID: 29946821
-
Coexisting JAK2V617F and CALR Exon 9 Mutations in Myeloproliferative Neoplasms - Do They Designate a New Subtype?Asian Pac J Cancer Prev. 2016;17(3):923-6. doi: 10.7314/apjcp.2016.17.3.923. Asian Pac J Cancer Prev. 2016. PMID: 27039813 Review.
-
The regulation of normal and neoplastic hematopoiesis is dependent on microenvironmental cells.Adv Biol Regul. 2018 Aug;69:11-15. doi: 10.1016/j.jbior.2018.06.003. Epub 2018 Jun 27. Adv Biol Regul. 2018. PMID: 29970351 Free PMC article. Review.
Cited by
-
IL-13/IL-4 signaling contributes to fibrotic progression of the myeloproliferative neoplasms.Blood. 2022 Dec 29;140(26):2805-2817. doi: 10.1182/blood.2022017326. Blood. 2022. PMID: 36283106 Free PMC article.
-
Potential Role of Sphingolipidoses-Associated Lysosphingolipids in Cancer.Cancers (Basel). 2022 Oct 5;14(19):4858. doi: 10.3390/cancers14194858. Cancers (Basel). 2022. PMID: 36230781 Free PMC article. Review.
-
Analysis of smoldering multiple myeloma according to the target of the monoclonal immunoglobulin of patients.Hemasphere. 2024 Dec 11;8(12):e70053. doi: 10.1002/hem3.70053. eCollection 2024 Dec. Hemasphere. 2024. PMID: 39670186 Free PMC article. No abstract available.
-
Neutrophil-specific expression of JAK2-V617F or CALRmut induces distinct inflammatory profiles in myeloproliferative neoplasia.J Hematol Oncol. 2024 Jun 9;17(1):43. doi: 10.1186/s13045-024-01562-5. J Hematol Oncol. 2024. PMID: 38853260 Free PMC article.
-
Progression of Myeloproliferative Neoplasms (MPN): Diagnostic and Therapeutic Perspectives.Cells. 2021 Dec 16;10(12):3551. doi: 10.3390/cells10123551. Cells. 2021. PMID: 34944059 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

