The Morphoregulatory Role of Thidiazuron: Metabolomics-Guided Hypothesis Generation for Mechanisms of Activity
- PMID: 32872300
- PMCID: PMC7564436
- DOI: 10.3390/biom10091253
The Morphoregulatory Role of Thidiazuron: Metabolomics-Guided Hypothesis Generation for Mechanisms of Activity
Abstract
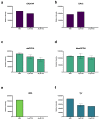
Thidiazuron (TDZ) is a diphenylurea synthetic herbicide and plant growth regulator used to defoliate cotton crops and to induce regeneration of recalcitrant species in plant tissue culture. In vitro cultures of African violet thin petiole sections are an ideal model system for studies of TDZ-induced morphogenesis. TDZ induces de novo shoot organogenesis at low concentrations and somatic embryogenesis at higher concentrations of exposure. We used an untargeted metabolomics approach to identify metabolites in control and TDZ-treated tissues. Statistical analysis including metabolite clustering, pattern and pathway tools, logical algorithms, synthetic biotransformations and hormonomics identified TDZ-induced changes in metabolism. A total of 18,602 putative metabolites with extracted masses and predicted formulae were identified with 1412 features that were found only in TDZ-treated tissues and 312 that increased in response to TDZ. The monomer of TDZ was not detected intact in the tissues but putative oligomers were found in the database and we hypothesize that these may form by a Diels-Alder reaction. Accumulation oligomers in the tissue may act as a reservoir, slowly releasing the active TDZ monomer over time. Cleavage of the amide bridge released TDZ-metabolites into the tissues including organic nitrogen and sulfur containing compounds. Metabolomics data analysis generated six novel hypotheses that can be summarized as an overall increase in uptake of sugars from the culture media, increase in primary metabolism, redirection of terpene metabolism and mediation of stress metabolism via indoleamine and phenylpropanoid metabolism. Further research into the specific mechanisms hypothesized is likely to unravel the mode of action of TDZ and to provide new insights into the control of plant morphogenesis.
Keywords: herbicide; mechanism of action; metabolomics; morphongenesis; phytohormone; plant growth and development; plant growth regulator; thidiazuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Mok M.C., Mok D.W.S., Armstrong D.J., Shudo K., Isogai Y., Okamoto T. Cytokinin activity of N-phenyl-N′-1, 2,3-thiadiazol-5-ylurea (thidiazuron) Phytochemistry. 1982;21:1509–1511. doi: 10.1016/S0031-9422(82)85007-3. - DOI
-
- Murthy B.N.S., Murch S.J., Saxena P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. Vitro Cell. Dev. Biol. Plant. 1998;34:267–275. doi: 10.1007/BF02822732. - DOI
-
- Xu J., Chen L., Sun H., Wusiman N., Sun W., Li B., Gao Y., Kong J., Zhang D., Zhang X., et al. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J. Exp. Bot. 2019;70:1525–1538. doi: 10.1093/jxb/erz036. - DOI - PMC - PubMed
-
- Xin F., Zhao J., Zhou Y., Wang G., Han X., Fu W., Deng J., Lan Y. Effects of Dosage and Spraying Volume on Cotton Defoliants Efficacy: A Case Study Based on Application of Unmanned Aerial Vehicles. Agronomy. 2018;8:85. doi: 10.3390/agronomy8060085. - DOI
-
- Xu J.H., Li C.P., Liu Z.S., Liu S.J., Zhang D.W., Ning X.M., Xie D.J. Breeding and planting techniques of the high-quality and high-yield new variety Xinluzao 50. China Cotton. 2011;38:35–36.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

