Transient Receptor Potential Canonical (TRPC) Channels: Then and Now
- PMID: 32872338
- PMCID: PMC7565274
- DOI: 10.3390/cells9091983
Transient Receptor Potential Canonical (TRPC) Channels: Then and Now
Abstract
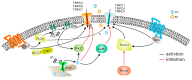
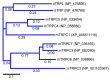
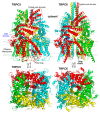
Twenty-five years ago, the first mammalian Transient Receptor Potential Canonical (TRPC) channel was cloned, opening the vast horizon of the TRPC field. Today, we know that there are seven TRPC channels (TRPC1-7). TRPCs exhibit the highest protein sequence similarity to the Drosophila melanogaster TRP channels. Similar to Drosophila TRPs, TRPCs are localized to the plasma membrane and are activated in a G-protein-coupled receptor-phospholipase C-dependent manner. TRPCs may also be stimulated in a store-operated manner, via receptor tyrosine kinases, or by lysophospholipids, hypoosmotic solutions, and mechanical stimuli. Activated TRPCs allow the influx of Ca2+ and monovalent alkali cations into the cytosol of cells, leading to cell depolarization and rising intracellular Ca2+ concentration. TRPCs are involved in the continually growing number of cell functions. Furthermore, mutations in the TRPC6 gene are associated with hereditary diseases, such as focal segmental glomerulosclerosis. The most important recent breakthrough in TRPC research was the solving of cryo-EM structures of TRPC3, TRPC4, TRPC5, and TRPC6. These structural data shed light on the molecular mechanisms underlying TRPCs' functional properties and propelled the development of new modulators of the channels. This review provides a historical overview of the major advances in the TRPC field focusing on the role of gene knockouts and pharmacological tools.
Keywords: TRPC; TRPC knockouts; TRPC modulators; calcium influx; cation channels.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

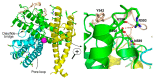


References
-
- Wong F., Hokanson K.M., Chang L.T. Molecular basis of an inherited retinal defect in Drosophila. Invest. Ophthalmol. Vis. Sci. 1985;26:243–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

