Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy
- PMID: 32872380
- PMCID: PMC7504748
- DOI: 10.3390/molecules25173945
Unravelling the Photoprotective Mechanisms of Nature-Inspired Ultraviolet Filters Using Ultrafast Spectroscopy
Abstract
There are several drawbacks with the current commercially available ultraviolet (UV) filters used in sunscreen formulations, namely deleterious human and ecotoxic effects. As a result of the drawbacks, a current research interest is in identifying and designing new UV filters. One approach that has been explored in recent years is to use nature as inspiration, which is the focus of this review. Both plants and microorganisms have adapted to synthesize their own photoprotective molecules to guard their DNA from potentially harmful UV radiation. The relaxation mechanism of a molecule after it has been photoexcited can be unravelled by several techniques, the ones of most interest for this review being ultrafast spectroscopy and computational methods. Within the literature, both techniques have been implemented on plant-, and microbial-inspired UV filters to better understand their photoprotective roles in nature. This review aims to explore these findings for both families of nature-inspired UV filters in the hope of guiding the future design of sunscreens.
Keywords: nature-inspired; photochemistry; photophysics; photoprotection; sunscreens; ultrafast spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




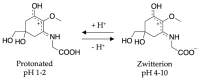
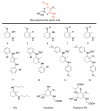

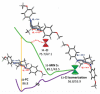
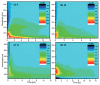

References
-
- Frederick J.E., Snell H.E., Haywood E.K. Solar ultraviolet radiation at the Earth’s surface. Photochem. Photobiol. 1989;50:443–450. doi: 10.1111/j.1751-1097.1989.tb05548.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

