What Is the Effectiveness of Different Duration Interdisciplinary Treatment Programs in Patients with Chronic Pain? A Large-Scale Longitudinal Register Study
- PMID: 32872448
- PMCID: PMC7564573
- DOI: 10.3390/jcm9092788
What Is the Effectiveness of Different Duration Interdisciplinary Treatment Programs in Patients with Chronic Pain? A Large-Scale Longitudinal Register Study
Abstract
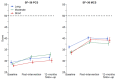
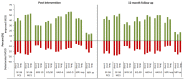
Chronic pain is a leading cause of disability globally. Interdisciplinary multimodal pain rehabilitation (IMPR) targets pain with a bio-psycho-social approach, often delivered as composite programs. However, evidence of optimal program duration for the rehabilitation to succeed remains scarce. This study evaluated the effectiveness of different duration IMPR-programs-using within- and between-effects analyses in a pragmatic multicenter register-based controlled design. Using the Swedish Quality Registry for Pain Rehabilitation, data from fifteen clinics specialized in chronic pain rehabilitation across Sweden were retrieved. Participants were patients with chronic musculoskeletal pain who had taken part in short (4-9 weeks; n = 924), moderate (10 weeks; n = 1379), or long (11-18 weeks; n = 395) IMPR programs. Longitudinal patient-reported outcome data were assessed at baseline, post-intervention, and at a 12-month follow-up. Primary outcomes were health-related quality of life, presented as perceived physical and mental health (SF-36). Secondary outcomes included the Hospital Anxiety and Depression Scale (HADS), pain intensity (NRS 0-10), the Multidimensional Pain Inventory (MPI), and perceived health (EQ-5D). Overall, all groups showed improvements. No clinically important effect emerged for different duration IMPR. In conclusion, while our results showed that patients following IMPR report improvement across a bio-psycho-social specter, a longer program duration was no more effective than a shorter one.
Keywords: multidisciplinary pain management; outcome measures; physical and mental functioning; practice-based evidence; registry study.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Merskey H., Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. IASP Press; Seattle, WA, USA: 1994. International Association for the Study of Pain. Task Force on, T.
-
- Turk D.C., Dworkin R.H., Revicki D., Harding G., Burke L.B., Cella D., Cleeland C.S., Cowan P., Farrar J.T., Hertz S., et al. Identifying important outcome domains for chronic pain clinical trials: An IMMPACT survey of people with pain. Pain. 2008;137:276–285. doi: 10.1016/j.pain.2007.09.002. - DOI - PubMed
-
- Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., Abdulkader R.S., Abdulle A.M., Abebo T.A., Abera S.F., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

