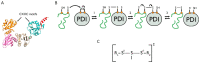Mechanisms of Disulfide Bond Formation in Nascent Polypeptides Entering the Secretory Pathway
- PMID: 32872499
- PMCID: PMC7565403
- DOI: 10.3390/cells9091994
Mechanisms of Disulfide Bond Formation in Nascent Polypeptides Entering the Secretory Pathway
Abstract
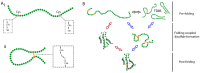
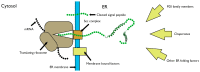
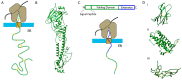
Disulphide bonds are an abundant feature of proteins across all domains of life that are important for structure, stability, and function. In eukaryotic cells, a major site of disulphide bond formation is the endoplasmic reticulum (ER). How cysteines correctly pair during polypeptide folding to form the native disulphide bond pattern is a complex problem that is not fully understood. In this paper, the evidence for different folding mechanisms involved in ER-localised disulphide bond formation is reviewed with emphasis on events that occur during ER entry. Disulphide formation in nascent polypeptides is discussed with focus on (i) its mechanistic relationship with conformational folding, (ii) evidence for its occurrence at the co-translational stage during ER entry, and (iii) the role of protein disulphide isomerase (PDI) family members. This review highlights the complex array of cellular processes that influence disulphide bond formation and identifies key questions that need to be addressed to further understand this fundamental process.
Keywords: ER; PDI; disulphide formation; protein folding; protein secretion; protein synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures