Neoplastic Cells are the Major Source of MT-MMPs in IDH1-Mutant Glioma, Thus Enhancing Tumor-Cell Intrinsic Brain Infiltration
- PMID: 32872536
- PMCID: PMC7565296
- DOI: 10.3390/cancers12092456
Neoplastic Cells are the Major Source of MT-MMPs in IDH1-Mutant Glioma, Thus Enhancing Tumor-Cell Intrinsic Brain Infiltration
Abstract
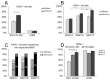
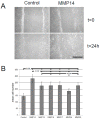
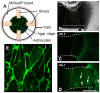
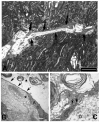
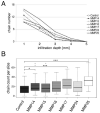
Tumor-cell infiltration is a major obstacle to successful therapy for brain tumors. Membrane-type matrix metalloproteinases (MT-MMPs), a metzincin subfamily of six proteases, are important mediators of infiltration. The cellular source of MT-MMPs and their role in glioma biology, however, remain controversial. Thus, we comprehensively analyzed the expression of MT-MMPs in primary brain tumors. All MT-MMPs were differentially expressed in primary brain tumors. In diffuse gliomas, MT-MMP1, -3, and -4 were predominantly expressed by IDH1mutated tumor cells, while macrophages/microglia contributed significantly less to MT-MMP expression. For functional analyses, individual MT-MMPs were expressed in primary mouse p53-/- astrocytes. Invasion and migration potential of MT-MMP-transduced astrocytes was determined via scratch, matrigel invasion, and novel organotypic porcine spinal slice migration (OPoSSM) and invasion assays. Overall, MT-MMP-transduced astrocytes showed enhanced migration compared to controls. MMP14 was the strongest mediator of migration in scratch assays. However, in the OPoSSM assays, the glycosylphosphatidylinositol (GPI)-anchored MT-MMPs MMP17 and MMP25, not MMP14, mediated the highest infiltration rates of astrocytes. Our data unequivocally demonstrate for the first time that glioma cells, not microglia, are the predominant producers of MT-MMPs in glioma and can act as potent mediators of tumor-cell infiltration into CNS tissue. These proteases are therefore promising targets for therapeutic interventions.
Keywords: glioma; invasion; isocitrate dehydrogenase; matrix metalloproteases; organotypic cell invasion assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Louis D., Ohgaki H., Wiestler O., Cavenee W. World Health Organization Histological Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer; Lion, France: 2016.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

