Innovative Molecular and Cellular Therapeutics in Cleft Palate Tissue Engineering
- PMID: 32873216
- PMCID: PMC8349724
- DOI: 10.1089/ten.TEB.2020.0181
Innovative Molecular and Cellular Therapeutics in Cleft Palate Tissue Engineering
Abstract
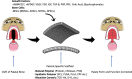
Clefts of the lip and/or palate are the most prevalent orofacial birth defects occurring in about 1:700 live human births worldwide. Early postnatal surgical interventions are extensive and staged to bring about optimal growth and fusion of palatal shelves. Severe cleft defects pose a challenge to correct with surgery alone, resulting in complications and sequelae requiring life-long, multidisciplinary care. Advances made in materials science innovation, including scaffold-based delivery systems for precision tissue engineering, now offer new avenues for stimulating bone formation at the site of surgical correction for palatal clefts. In this study, we review the present scientific literature on key developmental events that can go awry in palate development and the common surgical practices and challenges faced in correcting cleft defects. How key osteoinductive pathways implicated in palatogenesis inform the design and optimization of constructs for cleft palate correction is discussed within the context of translation to humans. Finally, we highlight new osteogenic agents and innovative delivery systems with the potential to be adopted in engineering-based therapeutic approaches for the correction of palatal defects. Impact statement Tissue-engineered scaffolds supplemented with osteogenic growth factors have attractive, largely unexplored possibilities to modulate molecular signaling networks relevant to driving palatogenesis in the context of congenital anomalies (e.g., cleft palate). Constructs that address this need may obviate current use of autologous bone grafts, thereby avoiding donor-site morbidity and other regenerative challenges in patients afflicted with palatal clefts. Combinations of biomaterials and drug delivery of diverse regenerative cues and biologics are currently transforming strategies exploited by engineers, scientists, and clinicians for palatal cleft repair.
Keywords: cleft palate; craniofacial; drug delivery; polymer scaffold; regenerative surgery; tissue engineering.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- McBride, W.A., McIntyre, G.T., Carroll, K., and Mossey, P.A.. Subphenotyping and classification of orofacial clefts: need for orofacial cleft subphenotyping calls for revised classification. Cleft Palate Craniofac J 53, 539, 2016 - PubMed
-
- Tanaka, S.A., Mahabir, R.C., Jupiter, D.C., and Menezes, J.M.. Updating the epidemiology of isolated cleft palate. Plast Reconstr Surg 131, 650e, 2013 - PubMed
-
- Tanaka, S.A., Mahabir, R.C., Jupiter, D.C., and Menezes, J.M.. Updating the epidemiology of cleft lip with or without cleft palate. Plast Reconstr Surg 129, 511e, 2012 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

