Prospective surveillance for intussusception in Indian children aged under two years at nineteen tertiary care hospitals
- PMID: 32873281
- PMCID: PMC7461288
- DOI: 10.1186/s12887-020-02293-5
Prospective surveillance for intussusception in Indian children aged under two years at nineteen tertiary care hospitals
Abstract
Background: India introduced rotavirus vaccines (RVV, monovalent, Rotavac™ and pentavalent, Rotasiil™) in April 2016 with 6, 10 and 14 weeks schedule and expanded countrywide in phases. We describe the epidemiology of intussusception among children aged 2-23 months in India.
Methods: The prospective surveillance at 19 nationally representative sentinel hospitals from four regions recruited children with intussusception from April 2016 to September 2017. Data on sociodemography, immunization, clinical, treatment and outcome were collected. Along with descriptive analysis, key parameters between four regions were compared using Chi-Square/Fisher's exact/Mann-Whitney U/Kruskal-Wallis tests. The pre- and post-RVV periods were compared to estimate the risk ratios.
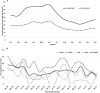
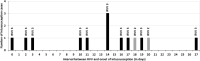
Results: Six hundred twenty-one children with intussusception from South (n = 262), East (n = 190), North (n = 136) and West (n = 33) regions were recruited. Majority (n = 465, 74.8%) were infants (40.0% aged 4-7 months) with median age 8 months (IQR 5, 13 months), predominantly males (n = 408, 65.7%) and half (n = 311, 50.0%) occurred during March-June months. A shorter interval between weaning and intussusception was observed for ragi based food (median 1 month, IQR 0-4.2 months) compared to rice (median 4 months, IQR 1-9 months) and wheat (median 3 months, IQR 1-7 months) based food (p < 0.01). Abdominal pain or excessive crying (82.8%), vomiting (72.6%), and bloody stool (58.1%) were the leading symptoms. Classical triad (abdominal pain, vomiting and bloody stool) was observed in 34.8% cases (24.4 to 45.8% across regions). 95.3% of the cases were diagnosed by ultrasound. 49.3% (10.5 to 82.4% across regions) cases were managed by reduction, 39.5% (11.5 to 71.1% across regions) cases underwent surgery and 11.1% spontaneously resolved. Eleven (1.8%) cases died. 89.1% cases met Brighton criteria level 1 and 7.6% met Level 2. RVV was received by 12 cases within 1-21 days prior to intussusception. No increase in case load (RR = 0.44; 95% CI 0.22-1.18) or case ratio (RR = 0.5; 95% CI 0.3-1.2) was observed after RVV introduction in select sites.
Conclusions: Intussusception cases were observed across all sites, although there were variations in cases, presentation and mode of management. The high case load age coincided with age of the RVV third dose. The association with ragi based weaning food in intussusception needs further evaluation.
Keywords: Children; Epidemiology; India; Intussusception; Prospective surveillance; Rotavirus vaccine; Sociodemography; Weaning food.
Conflict of interest statement
The authors declare that there is no competing interests and conflict of interest.
Figures
References
-
- Velázquez FR, Colindres RE, Grajales C, Hernández MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012;31(7):736–744. doi: 10.1097/INF.0b013e318253add3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

