COVID-19 Drive-Through Point of Screening and Testing (POST) System: A Safe, Efficient, and Adaptable Model for Nasopharyngeal Swab Collection
- PMID: 32873359
- PMCID: PMC7578630
- DOI: 10.1017/dmp.2020.313
COVID-19 Drive-Through Point of Screening and Testing (POST) System: A Safe, Efficient, and Adaptable Model for Nasopharyngeal Swab Collection
Abstract
Objective: The authors aim to demonstrate that the current drive-through testing model at a health district was improved in certain parameters compared with a previous testing protocol, and to provide the methodology of the current model for other coronavirus disease (COVID-19) testing sites to potentially emulate.
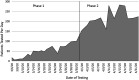
Methods: Initially, a small drive-through site was constructed at a converted tuberculosis clinic, but due to an increase in testing needs, an expanded point of screening and testing (POST) system was developed in an event center parking lot to administer tests to a higher volume of patients.
Results: An average of 51.1 patients was tested each day (2.0 tests per personnel in personal protective equipment [PPE] per hour) at the initial tuberculosis clinic drive-through site, which increased to 217.8 patients tested each day (5.9 tests per personnel in PPE per hour) with the new drive-through POST system (P < 0.001). Mean testing time was 3.4 minutes and the total time on-site averaged 14.4 minutes.
Conclusions: This POST drive-through system serves as an efficient, safe, and adaptable model for high volume COVID-19 nasopharyngeal swabbing that the authors recommend other COVID-19 testing sites nationwide consider adopting for their own use.
Keywords: COVID-19; coronavirus; drive-through; health district; screening.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Willingness to Use Home Collection Methods to Provide Specimens for SARS-CoV-2/COVID-19 Research: Survey Study.J Med Internet Res. 2020 Sep 3;22(9):e19471. doi: 10.2196/19471. J Med Internet Res. 2020. PMID: 32790639 Free PMC article.
-
Saliva Testing Is Accurate for Early-Stage and Presymptomatic COVID-19.Microbiol Spectr. 2021 Sep 3;9(1):e0008621. doi: 10.1128/Spectrum.00086-21. Epub 2021 Jul 14. Microbiol Spectr. 2021. PMID: 34259552 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Discrepancies of SARS-CoV-2 testing results among patients with total laryngectomy.Eur Arch Otorhinolaryngol. 2022 Apr;279(4):2193-2196. doi: 10.1007/s00405-021-07203-8. Epub 2021 Dec 2. Eur Arch Otorhinolaryngol. 2022. PMID: 34853865 Free PMC article. Review.
-
Complications of Nasopharyngeal Swabs and Safe Procedures for COVID-19 Testing Based on Anatomical Knowledge.J Korean Med Sci. 2022 Mar 21;37(11):e88. doi: 10.3346/jkms.2022.37.e88. J Korean Med Sci. 2022. PMID: 35315599 Free PMC article. Review.
Cited by
-
Agent Simulation Model of COVID-19 Epidemic Agent-Based on GIS: A Case Study of Huangpu District, Shanghai.Int J Environ Res Public Health. 2022 Aug 18;19(16):10242. doi: 10.3390/ijerph191610242. Int J Environ Res Public Health. 2022. PMID: 36011877 Free PMC article.
-
High-Resolution Agent-Based Modeling of COVID-19 Spreading in a Small Town.Adv Theory Simul. 2021 Mar;4(3):2000277. doi: 10.1002/adts.202000277. Epub 2021 Jan 18. Adv Theory Simul. 2021. PMID: 33786413 Free PMC article.
-
Viral Etiological Agent(s) of Respiratory Tract Infections in Symptomatic Individuals during the Second Wave of COVID-19 Pandemic: A Single Drive-Thru Mobile Collection Site Study.Pathogens. 2022 Apr 15;11(4):475. doi: 10.3390/pathogens11040475. Pathogens. 2022. PMID: 35456150 Free PMC article.
-
A systematic review of strategies adopted to scale up COVID-19 testing in low-, middle- and high-income countries.BMJ Open. 2022 Nov 17;12(11):e060838. doi: 10.1136/bmjopen-2022-060838. BMJ Open. 2022. PMID: 36396316 Free PMC article.
-
Analyzing factors affecting positivity in drive-through COVID-19 testing: a cross-sectional study.Virol J. 2024 May 14;21(1):111. doi: 10.1186/s12985-024-02388-w. Virol J. 2024. PMID: 38745200 Free PMC article.
References
-
- Trump D. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. United States: The White House; 2020.
-
- United States: National Conference of State Legislatures. State action on coronavirus (COVID-19). 2020. https://www.ncsl.org/research/health/state-action-on-coronavirus-covid-1.... Accessed April 19, 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

