Negative Interplay between Biofilm Formation and Competence in the Environmental Strains of Bacillus subtilis
- PMID: 32873610
- PMCID: PMC7470987
- DOI: 10.1128/mSystems.00539-20
Negative Interplay between Biofilm Formation and Competence in the Environmental Strains of Bacillus subtilis
Abstract
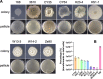
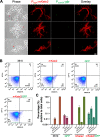
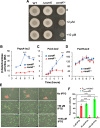
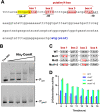
Environmental strains of the soil bacterium Bacillus subtilis have valuable applications in agriculture, industry, and biotechnology; however, environmental strains are genetically less accessible. This reduced accessibility is in sharp contrast to laboratory strains, which are well known for their natural competence, and a limitation in their applications. In this study, we observed that robust biofilm formation by environmental strains of B. subtilis greatly reduced the frequency of competent cells in the biofilm. By using model strain 3610, we revealed a cross-pathway regulation that allows biofilm matrix producers and competence-developing cells to undergo mutually exclusive cell differentiation. We further demonstrated that the competence activator ComK represses the key biofilm regulatory gene sinI by directly binding to the sinI promoter, thus blocking competent cells from simultaneously becoming matrix producers. In parallel, the biofilm activator SlrR represses competence through three distinct mechanisms involving both genetic regulation and cell morphological changes. Finally, we discuss the potential implications of limiting competence in a bacterial biofilm.IMPORTANCE The soil bacterium Bacillus subtilis can form robust biofilms, which are important for its survival in the environment. B. subtilis also exhibits natural competence. By investigating competence development in B. subtilis in situ during biofilm formation, we reveal that robust biofilm formation often greatly reduces the frequency of competent cells within the biofilm. We then characterize a cross-pathway regulation that allows cells in these two developmental events to undergo mutually exclusive cell differentiation during biofilm formation. Finally, we discuss potential biological implications of limiting competence in a bacterial biofilm.
Keywords: Bacillus subtilis; biofilm; cell differentiation; competence; environmental strains.
Copyright © 2020 She et al.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

