Prospective multicentre randomised trial comparing the efficacy and safety of single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) versus Roux-en-Y gastric bypass (RYGB): SADISLEEVE study protocol
- PMID: 32873678
- PMCID: PMC7467507
- DOI: 10.1136/bmjopen-2020-037576
Prospective multicentre randomised trial comparing the efficacy and safety of single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) versus Roux-en-Y gastric bypass (RYGB): SADISLEEVE study protocol
Abstract
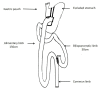
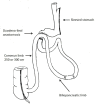
Introduction: Despite the non-negligible weight loss failure rate at midterm, Roux-en-Y gastric bypass (RYGB) remains the reference procedure in the treatment of morbid obesity with metabolic comorbidities. A recently emerged procedure, the single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S), could be more effective on weight loss with similar morbidity and lower weight loss failure rate than RYGB. We propose the first randomised, open, multicentre superiority trial comparing the SADI-S to RYGB (SADISLEEVE).
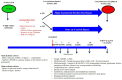
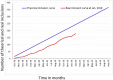
Methods and analysis: The main objective is to demonstrate the superiority at 2 years after surgery of the SADI-S compared with RYGB in term of excess weight loss percentage. The secondary objectives are the evaluation of nutritional status, metabolic outcomes, overall complication rates and quality of life, within 2 years after surgery. Key inclusion criteria are obese patients with body mass index (BMI) ≥40 kg/m2 or ≥35 kg/m2 with at least one comorbid condition and candidate to a first bariatric procedure or after failure of sleeve gastrectomy. Patients randomised by minimisation in two arms, based on centre, surgery as a revisional procedure, presence of type 2 diabetes and BMI >50 kg/m2 will be included over 2 years.A sample size of 166 patients in each group will have a power of 90% to detect a probability of 0.603 that excess weight loss in the RYGB arm is less than excess weight loss in the SADI-S arm with a 5% two-sided significance level. With a drop-out rate of 10%, it will be necessary to include 183 patients per group.
Ethics and dissemination: The study was approved by Institutional Review Board of Centre Hospitalier Universitaire Morvan (CPP1089-HPS1). Study was also approved by the French national agency for drug safety (2018061500148). Results will be reported in peer-reviewed scientific journals.
Trial registration number: NCT03610256.
Keywords: clinical trials; nutrition & dietetics; surgery.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DM-B reports personal fees from Maat Pharma outside of the submitted work. MR reports fees as a consultant from Medtronic and fees as an expert speaker from Gore outside of the submitted work.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
