Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma
- PMID: 32873681
- PMCID: PMC7467531
- DOI: 10.1136/bmjopen-2020-038892
Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma
Abstract
Introduction: Mesothelioma remains a lethal cancer. To date, systemic therapy with pemetrexed and a platinum drug remains the only licensed standard of care. As the median survival for patients with mesothelioma is 12.1 months, surgery is an important consideration to improve survival and/or quality of life. Currently, only two surgical trials have been performed which found that neither extensive (extra-pleural pneumonectomy) or limited (partial pleurectomy) surgery improved survival (although there was some evidence of improved quality of life). Therefore, clinicians are now looking to evaluate pleurectomy decortication, the only radical treatment option left.
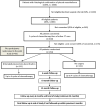
Methods and analysis: The MARS 2 study is a UK multicentre open parallel group randomised controlled trial comparing the effectiveness and cost-effectiveness of surgery-(extended) pleurectomy decortication-versus no surgery for the treatment of pleural mesothelioma. The study will test the hypothesis that surgery and chemotherapy is superior to chemotherapy alone with respect to overall survival. Secondary outcomes include health-related quality of life, progression-free survival, measures of safety (adverse events) and resource use to 2 years. The QuinteT Recruitment Intervention is integrated into the trial to optimise recruitment.
Ethics and dissemination: Research ethics approval was granted by London - Camberwell St. Giles Research Ethics Committee (reference 13/LO/1481) on 7 November 2013. We will submit the results for publication in a peer-reviewed journal.
Trial registration numbers: ISRCTN-ISRCTN44351742 and ClinicalTrials.gov-NCT02040272.
Keywords: chemotherapy; oncology; thoracic medicine; thoracic surgery.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Health and Safety Executive Asbestos-related disease statistics in Great Britain, 2019, 2019. https://www.hse.gov.uk/statistics/causdis/asbestos-related-disease.pdf
-
- National Institute of Health and Care Excellence Pemetrexed for the treatment of malignant pleural mesothelioma. Technology appraisal guidance [TA135], 2008.
-
- Royal College of Physicians National lung cancer audit pleural mesothelioma report 2016 (for the audit period 2014). London: Royal College of Physicians, 2016.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous