Identification of compounds that bind the centriolar protein SAS-6 and inhibit its oligomerization
- PMID: 32873708
- PMCID: PMC7939395
- DOI: 10.1074/jbc.RA120.014780
Identification of compounds that bind the centriolar protein SAS-6 and inhibit its oligomerization
Abstract
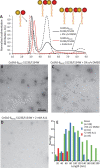
Centrioles are key eukaryotic organelles that are responsible for the formation of cilia and flagella, and for organizing the microtubule network and the mitotic spindle in animals. Centriole assembly requires oligomerization of the essential protein spindle assembly abnormal 6 (SAS-6), which forms a structural scaffold templating the organization of further organelle components. A dimerization interaction between SAS-6 N-terminal "head" domains was previously shown to be essential for protein oligomerization in vitro and for function in centriole assembly. Here, we developed a pharmacophore model allowing us to assemble a library of low-molecular-weight ligands predicted to bind the SAS-6 head domain and inhibit protein oligomerization. We demonstrate using NMR spectroscopy that a ligand from this family binds at the head domain dimerization site of algae, nematode, and human SAS-6 variants, but also that another ligand specifically recognizes human SAS-6. Atomistic molecular dynamics simulations starting from SAS-6 head domain crystallographic structures, including that of the human head domain which we now resolve, suggest that ligand specificity derives from favorable Van der Waals interactions with a hydrophobic cavity at the dimerization site.
Keywords: biophysics; centrosome; inhibitor; ligand design; nuclear magnetic resonance (NMR); protein-drug interaction; protein-protein interaction.
© 2020 Busch et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures
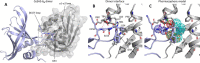



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

