Lysine acetylation of F-actin decreases tropomyosin-based inhibition of actomyosin activity
- PMID: 32873710
- PMCID: PMC7667958
- DOI: 10.1074/jbc.RA120.015277
Lysine acetylation of F-actin decreases tropomyosin-based inhibition of actomyosin activity
Abstract
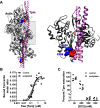
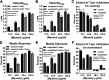
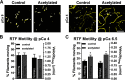
Recent proteomics studies of vertebrate striated muscle have identified lysine acetylation at several sites on actin. Acetylation is a reversible post-translational modification that neutralizes lysine's positive charge. Positively charged residues on actin, particularly Lys326 and Lys328, are predicted to form critical electrostatic interactions with tropomyosin (Tpm) that promote its binding to filamentous (F)-actin and bias Tpm to an azimuthal location where it impedes myosin attachment. The troponin (Tn) complex also influences Tpm's position along F-actin as a function of Ca2+ to regulate exposure of myosin-binding sites and, thus, myosin cross-bridge recruitment and force production. Interestingly, Lys326 and Lys328 are among the documented acetylated residues. Using an acetic anhydride-based labeling approach, we showed that excessive, nonspecific actin acetylation did not disrupt characteristic F-actin-Tpm binding. However, it significantly reduced Tpm-mediated inhibition of myosin attachment, as reflected by increased F-actin-Tpm motility that persisted in the presence of Tn and submaximal Ca2+ Furthermore, decreasing the extent of chemical acetylation, to presumptively target highly reactive Lys326 and Lys328, also resulted in less inhibited F-actin-Tpm, implying that modifying only these residues influences Tpm's location and, potentially, thin filament regulation. To unequivocally determine the residue-specific consequences of acetylation on Tn-Tpm-based regulation of actomyosin activity, we assessed the effects of K326Q and K328Q acetyl (Ac)-mimetic actin on Ca2+-dependent, in vitro motility parameters of reconstituted thin filaments (RTFs). Incorporation of K328Q actin significantly enhanced Ca2+ sensitivity of RTF activation relative to control. Together, our findings suggest that actin acetylation, especially Lys328, modulates muscle contraction via disrupting inhibitory Tpm positioning.
Keywords: acetylation; actin; in vitro motility; lysine acetylation; muscle physiology; myosin; thin filament; tropomyosin.
© 2020 Schmidt et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

